Abstract
Purpose: Surgical stress has been suggested to facilitate the growth of preexisting micrometastases as well as small residual tumor postoperatively. The purpose of this study was to examine the effects of surgical stress on ovarian cancer growth and to determine underlying mechanisms responsible for increased growth.
Experimental Design: To mimic the effects of surgery, we did a laparotomy or mastectomy under isoflurane inhalation on athymic nude mice 4 days after i.p. tumor cell injection. Propranolol infusion via Alzet pumps was used to block the influence of sympathetic nervous system activation by surgical stress.
Results: In both HeyA8 and SKOV3ip1 models, the mice in the laparotomy and mastectomy groups had significantly greater tumor weight (P < 0.05) and nodules (P < 0.05) compared with anesthesia only controls. There was no increase in tumor weight following surgery in the β-adrenergic receptor–negative RMG-II model. Propranolol completely blocked the effects of surgical stress on tumor growth, indicating a critical role for β-adrenergic receptor signaling in mediating the effects of surgical stress on tumor growth. In the HeyA8 and SKOV3ip1 models, surgery significantly increased microvessel density (CD31) and vascular endothelial growth factor expression, which were blocked by propranolol treatment.
Conclusion: These results indicate that surgical stress could enhance tumor growth and angiogenesis, and β-blockade might be effective in preventing such effects.
Surgical cytoreduction is considered a critical component of cancer therapy for many tumor types, including ovarian cancer. Some surgeons have observed rapid tumor regrowth shortly after primary surgery. Moreover, women with ovarian cancer frequently undergo several surgical procedures throughout the course of their disease. Surgical stress has been suggested to facilitate the growth of preexisting micrometastases as well as small residual tumor postoperatively. The key findings from our study are that increased angiogenic processes mediated by surgical stress could promote ovarian cancer growth in vivo. This increase in tumor growth could be completely blocked by a β-adrenergic receptor antagonist (propranolol). These findings could have significant implications for the perioperative management of cancer patients to prevent surgical stress–induced tumor growth and angiogenesis.
Surgical cytoreduction is a critical component of cancer therapy for many tumor types, including ovarian cancer. In clinical practice, outgrowth of distant metastasis has been described following surgical removal of primary tumors (1). This observation has been further supported by several models that have shown increased tumor growth and metastatic spread following surgery (2–7). There are several potential mechanisms for the effects on tumor growth by surgical stress, including shedding of tumor cells due to physical manipulation (8, 9), a drop in the level of antiangiogenic factors (10), local and systemic release of growth factors or cytokines (11), and suppression of cell-mediated immunity (12). However, the mechanisms by which surgical stress promotes tumor growth are not fully understood.
To date, no reliable screening methods to detect ovarian cancer at an early stage have been developed. Thus, ∼70% of women with newly discovered ovarian cancer have advanced disease at the time of diagnosis (13). Nevertheless, a complete clinical remission can be achieved in ∼80% of these patients with the use of maximal surgical cytoreduction and platinum-based combination chemotherapy (14). Unfortunately, upwards of 75% of those with complete clinical response will develop recurrent disease. Reoperation with secondary tumor resection has become an option for many ovarian cancer patients. Therefore, women with ovarian cancer frequently undergo several surgical procedures throughout the course of their disease. Given the paucity of information about the effects of surgery on ovarian cancer growth, we examined the effects of laparotomy and extraperitoneal surgery on ovarian cancer growth and angiogenesis using orthotopic animal models.
Materials and Methods
Ovarian cell lines and culture conditions. The ovarian cancer cell lines HeyA8, SKOV3ip1, and RMG-II were cultured in RPMI 1640 supplemented with 10% (RMG-II) to 15% (HeyA8 and SKOV3ip1) fetal bovine serum and 0.1% gentamicin sulfate (Gemini Bioproducts; refs. 15–17). For in vivo injections, cells were trypsinized and centrifuged at 1,000 rpm for 7 min at 4°C, washed twice with PBS, and reconstituted in serum-free HBSS (Life Technologies/Invitrogen). Only single-cell suspensions with >95% viability, as determined by trypan blue exclusion, were used for in vivo injections. All experiments were done using cells grown to 60% to 80% confluence, and all cell lines were routinely tested to confirm absence of Mycoplasma.
Animal care and orthotopic implantation of tumor cells. Female athymic mice (NCr-nu) were purchased from the National Cancer Institute-Frederick Cancer Research and Development Center. The mice were housed and maintained under specific pathogen-free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with the current regulations and standards of the U.S. Department of Agriculture, the U.S. Department of Health and Human Services, and the NIH. All studies were approved and supervised by the University of Texas M. D. Anderson Cancer Center Institutional Animal Care and Use Committee. To produce tumors, HeyA8 (2.5 × 105 cells/0.2 mL HBSS), SKOV3ip1 (1.0 × 106 cells/0.2 mL HBSS), or RMG-II (5.0 × 106 cells/0.2 mL HBSS) were injected i.p. into the peritoneal cavity of mice (15–17). The number of cells injected for each cell line was established previously based on the lowest number of cells required to reliably produce tumors. The mice used in these experiments were 8 to 12 wk old. Mice (n = 10 per group) were monitored daily for tumor development and postoperative complications and were sacrificed on days 25 to 30 (HeyA8), days 35 to 40 (SKOV3ip1), or days 40 to 50 (RMG-II) or when any of the mice seemed moribund. Total body weight, tumor incidence and mass, and the number of tumor nodules were recorded. Tumors were fixed in formalin and embedded in paraffin or snap frozen in OCT compound (Sakura Finetek USA, Inc.) in liquid nitrogen.
Generation of orthotopic in vivo model of surgical stress. For surgery, animals were exposed to an experimental mastectomy or laparotomy under isoflurane inhalation (Baxter) anesthesia. The surgical procedure for mastectomy consisted of a midline chest wall skin incision and removal of right mammary tissues from the chest wall. The skin of chest wall was closed with three to four surgical clips. The laparotomy consisted of a 4-cm midline abdominal incision followed by the externalization of intestines for a period of 4 min as described previously by Ben-Eliyahu and colleagues (18, 19). During the laparotomy, the small intestine was rubbed with two saline-soaked cotton Q-tips in four locations to simulate a surgical procedure. The intestine was then returned to the abdominal cavity and irrigated with saline, and the abdominal wall was closed with surgical clips (20, 21).
To examine the influence of β-adrenergic signaling for surgical stress, we used the nonspecific β-adrenergic receptor (ADRB) antagonist (S)-propranolol hydrochloride (2 mg/kg/d; Sigma) given via Alzet mini-osmotic pumps (model 2004, DURECT Corp.; refs. 22, 23). We inserted the minipumps containing PBS or propranolol on the nape of neck 7 d before surgical stress (24).
Immunohistochemistry for CD31, vascular endothelial growth factor, and proliferating cell nuclear antigen. To quantify angiogenesis, microvessel density (MVD) was ascertained by counting CD31-positive vessels as described previously (15). In brief, 8-μm sections of fresh-frozen samples were fixed and incubated with anti-mouse CD31 (1:800; Pharmingen) at 4°C overnight. Immunohistochemical procedures for vascular endothelial growth factor (VEGF) and proliferating cell nuclear antigen (PCNA) were done as described previously (25). In brief, for detecting VEGF and PCNA immunoreactivity, formalin-fixed, paraffin-embedded serial sections were deparaffinized by sequential washes with xylene followed by descending grades of ethanol. Depending on the antibody used, antigen retrieval was achieved by either citrate buffer (pH 6.0) in a steamer (PCNA) or pepsin in a 37°C humidified incubator (VEGF). Endogenous peroxidases were blocked with 3% H2O2 in methanol. Nonspecific proteins and exposed epitopes were blocked with 5% normal horse serum/1% normal goat serum, and slides were incubated at 4°C overnight with the respective primary antibody at the following dilutions: 1:100 VEGF (Santa Cruz Biotechnology) or 1:50 PCNA (Dako). After PBS washing, the appropriate secondary antibody was applied for 1 h at room temperature. Visualization was achieved with 3,3′-diaminobenzidine chromogen. All counterstaining was done with Gill's hematoxylin (Sigma-Aldrich).
Microscopic quantitative analyses of MVD and PCNA. To quantify MVD, 10 random fields at ×100 magnification per slide were examined for each tumor (1 slide per mouse, 5 slides per treatment group) and the number of microvessels per field was counted by two investigators (J-W.L. and H-S.K.) in a blinded fashion. A single microvessel was defined as a discrete cluster or single cell stained positive for CD31 with the presence of a lumen (17). To quantify PCNA expression, the number of PCNA-positive cells and the total number of tumor cells were counted in five random fields at ×100 magnification followed by calculation of the percentage of positive cells (17).
Measurement of cytokines/chemokines in serum of mice after surgical stress. To check the cytokines and chemokines in serum of mice, we used multiplexing xMAP technology to analyze mouse Cytokine/Chemokine 17 Plex Panel (Bio-Rad Laboratories) that included granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), IFN-γ, interleukin (IL)-1α, IL-1β, IL-6, IL-10, IL-12 (p70), IL-15, MCP-1, M-CSF, MIG, macrophage inflammatory protein (MIP)-1α, MIP-1β, MIP-2, RANTES, and tumor necrosis factor α (TNFα). Blood samples were taken from hearts during inhalation anesthesia to minimize further stress. Serum samples were also obtained from tumor-bearing mice 6 h, 1 d, 3 d, and 5 d later after laparotomy (n = 2 per group). Appropriate control samples were obtained from mice exposed to isoflurane inhalation alone. Multiplex xMAP assays were done according to the manufacturer's protocols. Samples were analyzed using the Bio-Plex suspension array system (Bio-Rad Laboratories). For each analyte, 100 beads were analyzed and means were calculated. Analysis of experimental data was done using four-parametric curve fitting to the standard analyte curves.
Statistical methods. Continuous variables were compared with the Student's t test or ANOVA if normally distributed and the Mann-Whitney rank sum test if distributions were nonparametric using Statistical Package for the Social Sciences (SPSS, Inc.). A P value of <0.05 was considered statistically significant.
Results
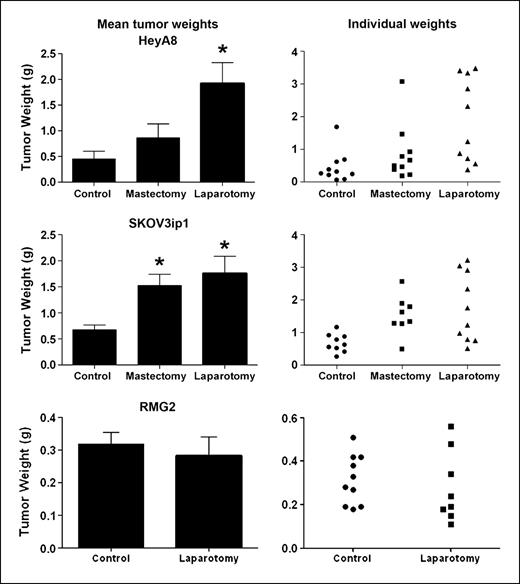
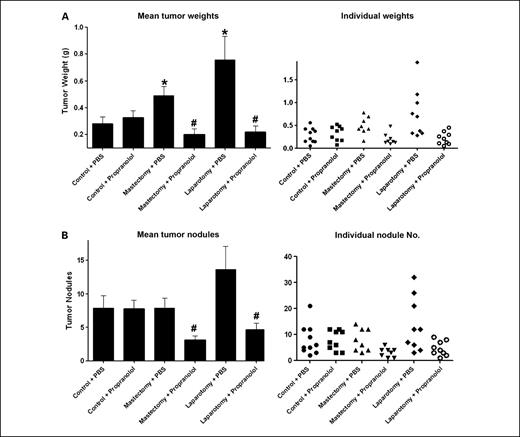
Effect of surgical stress on tumor growth. To mimic the effects of surgery, we did a laparotomy on mice 4 days after tumor cell injection. In addition, to account for possibility of local effects, we also did a mastectomy on a separate group of animals. In the ADRB-positive HeyA8 and SKOV3ip1 models, laparotomy resulted in 370% (HeyA8) and 263% (SKOV3ip1) increases in tumor weight (P < 0.05 for both models; Fig. 1). Mastectomy also increased tumor weight by 203% (HeyA8) and 226% (SKOV3ip1; P < 0.05) compared with anesthesia only controls (Fig. 1). Similarly, the number of tumor nodules was also significantly greater following a laparotomy in both models (P < 0.05; data not shown). Because the sympathetic nervous system is activated during surgery (19, 26), we next asked whether tumor cells with ADRB expression might be responsible for the increased tumor growth. To address this question, we first used an ADRB-null model using the RMG-II cells that were negative for both ADRB1 and ADRB2, as previously shown by reverse transcription-PCR, Western blot, and lack of intracellular cyclic AMP response to norepinephrine or isoproterenol (24). In this model, there was no difference in tumor weight between the control and laparotomy groups (0.32 versus 0.28 g; P = 0.42; Fig. 1). There were no significant differences in the body weight of animals between the groups, suggesting that surgery did not adversely affect the overall well-being of the animals. Next, we examined whether a β-blocker would abrogate the increased tumor growth. For these experiments, we used the SKOV3ip1 model and inserted a mini-osmotic pump containing propranolol into the mice to deliver the drug continuously. There were no significant complications associated with pump insertion into the mice. Figure 2 shows that either mastectomy or laparotomy resulted in significantly increased tumor weight compared with controls in the group with PBS-containing pumps. Treatment with propranolol had no significant effect on tumor growth in the anesthesia only controls. However, treatment with continuous propranolol infusion was able to block the increased tumor growth observed in response to either mastectomy or laparotomy (Fig. 2).
Effect of surgical stress on in vivo ovarian cancer growth. Quantification of tumor weights in control (anesthesia alone) mice and in surgically stressed mice (mastectomy or laparotomy) that were injected i.p. with HeyA8, SKOV3ip1, or RMG-II ovarian cancer cells. Results represent the mean ± SE; n = 10 mice per group. *, P < 0.05, compared with control group.
Effect of surgical stress on in vivo ovarian cancer growth. Quantification of tumor weights in control (anesthesia alone) mice and in surgically stressed mice (mastectomy or laparotomy) that were injected i.p. with HeyA8, SKOV3ip1, or RMG-II ovarian cancer cells. Results represent the mean ± SE; n = 10 mice per group. *, P < 0.05, compared with control group.
Effect of propranolol (β-antagonist) in the SKOV3ip1 model for surgical stress. Tumor weight (A) and the number of nodules (B) following treatment with the propranolol (2 mg/kg/d) using osmotic minipumps. Results represent the mean ± SE; n = 10 mice per group. *, P < 0.05, compared with control group; #, P < 0.05, compared with each stress + PBS group.
Effect of propranolol (β-antagonist) in the SKOV3ip1 model for surgical stress. Tumor weight (A) and the number of nodules (B) following treatment with the propranolol (2 mg/kg/d) using osmotic minipumps. Results represent the mean ± SE; n = 10 mice per group. *, P < 0.05, compared with control group; #, P < 0.05, compared with each stress + PBS group.
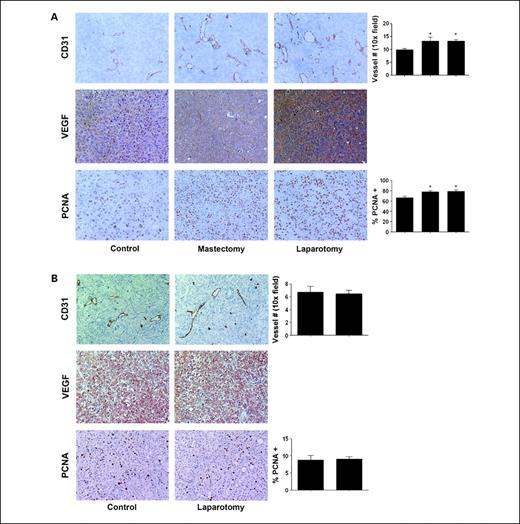
Effect of surgical stress on angiogenesis and cell proliferation. Based on our prior findings related to the effects of stress hormones on tumor growth and angiogenesis (24), we considered whether tumor angiogenesis might be stimulated following surgery. When we examined MVD in the harvested tumors, surgical stress resulted in significantly greater MVD counts (P < 0.05) in the HeyA8 (Fig. 3A) and SKOV3ip1 (Supplementary Fig. S1) models. But these findings were not present in the ADRB-null RMG-II model (Fig. 3B). Moreover, surgical stress showed increased VEGF staining compared with controls in the HeyA8 (Fig. 3A) and SKOV3ip1 (Supplementary Fig. S1) models. There was no difference in VEGF expression in the RMG-II model (Fig. 3B). We also examined the effects of surgery on tumor cell proliferation using PCNA staining. In the HeyA8 (Fig. 3A) and SKOV3ip1 (Supplementary Fig. S1) models, positive staining of PCNA expression was significantly increased in the tumor following a mastectomy or laparotomy compared with controls (P < 0.05, both). But in the RMG-II model, there was no significant difference between the two groups (Fig. 3B).
Proliferation and angiogenesis in tumor tissues is increased in tumors harvested from animals exposed to surgical stress. A, HeyA8 tumor samples from control (anesthesia alone) or surgically (mastectomy or laparotomy) stressed animals were stained for CD31, VEGF, and PCNA by immunohistochemistry. B, RMG-II (ADRB null) tumor samples from mice exposed to surgery did not show any differences in CD31, VEGF, and PCNA staining between control and laparotomy. All photographs were taken at original magnification ×100. The bars in the graphs correspond sequentially to the labeled columns of images at left. Bars, SE. *, P < 0.05.
Proliferation and angiogenesis in tumor tissues is increased in tumors harvested from animals exposed to surgical stress. A, HeyA8 tumor samples from control (anesthesia alone) or surgically (mastectomy or laparotomy) stressed animals were stained for CD31, VEGF, and PCNA by immunohistochemistry. B, RMG-II (ADRB null) tumor samples from mice exposed to surgery did not show any differences in CD31, VEGF, and PCNA staining between control and laparotomy. All photographs were taken at original magnification ×100. The bars in the graphs correspond sequentially to the labeled columns of images at left. Bars, SE. *, P < 0.05.
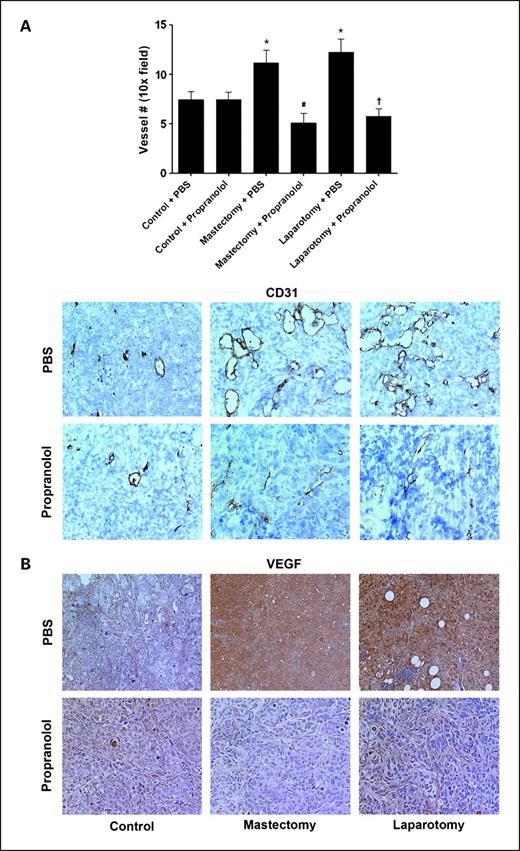
To determine the effect of propranolol on angiogenesis in surgical stress, we did immunohistochemistry for angiogenesis markers in tumor samples. Increased MVD and VEGF by surgery were blocked by propranolol (Fig. 4).
The effect of propranolol on angiogenesis in surgically stressed mice. SKOV3ip1 tumor samples obtained from control or surgically stressed mice were treated with placebo (PBS) or propranolol pump and stained for CD31 (A) and VEGF (B) by immunohistochemistry. All photographs were taken at original magnification ×100. Bars, SE. *, P < 0.05.
The effect of propranolol on angiogenesis in surgically stressed mice. SKOV3ip1 tumor samples obtained from control or surgically stressed mice were treated with placebo (PBS) or propranolol pump and stained for CD31 (A) and VEGF (B) by immunohistochemistry. All photographs were taken at original magnification ×100. Bars, SE. *, P < 0.05.
Level of cytokine/chemokine in mouse serum after surgical stress. It is likely that other cytokines may be activated during the physiologic response to surgery. Therefore, we examined a panel of 17 cytokines and chemokines, including G-CSF, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-6, IL-10, IL-12 (p70), IL-15, MCP-1, M-CSF, MIG, MIP-1α, MIP-1β, MIP-2, RANTES, and TNFα, in mouse serum after surgical stress. Serum samples were obtained from tumor-bearing mice with control (anesthesia with isoflurane inhalation alone) 6 hours, 1 day, 3 days, and 5 days later after laparotomy (n = 2 of each group). Concentrations of G-CSF, IL-1α, IL-6, and IL-15 were significantly higher during the perioperative period (Fig. 5).
Levels of cytokines/chemokines in mouse serum following surgical stress. Cytokine/chemokine concentrations were measured in mouse sera using xMAP technology with multiplex cytokine Bio-Rad kit. Columns, mean; bars, SE.
Levels of cytokines/chemokines in mouse serum following surgical stress. Cytokine/chemokine concentrations were measured in mouse sera using xMAP technology with multiplex cytokine Bio-Rad kit. Columns, mean; bars, SE.
Discussion
The key findings from our study are that increased angiogenic processes mediated by surgical stress could promote ovarian cancer growth in vivo. The greater tumor growth was associated with increased angiogenesis and could be completely blocked by ADRB blockade. Based on these findings, perioperative use of a β-blocker could have preventive effects for surgical stress–induced tumor growth in ovarian cancer patients.
Surgery, as a stressor, could increase the risk of postoperative metastases and their underlying signaling mechanisms via local or systemic effects. Some reports have suggested that a surgical wound has important roles in mediating the effects of surgical stress as a source of tumor-stimulating factors (11, 27, 28). These reports suggested that growth factors or cytokines released by wounding could contribute to creating a suitable environment for tumor growth (11). Wound healing and tumor progression both involve processes of cell proliferation, inflammation, and angiogenesis. In contrast to local effects, Ben-Eliyahu et al. suggested that surgical stress results in systemic activation of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system, resulting in suppressed natural killer cell response to tumor cells (29, 30). They also found that combined use of a β-blocker and a prostaglandin synthesis inhibitor restored natural killer function (19).
We recently reported that chronic (immobilization) stress promotes tumor development and progression via β-adrenergic activation of the cyclic AMP–protein kinase A signaling pathways in an orthotopic murine model of ovarian carcinoma (24). In addition to stress, understanding the mechanisms responsible for mediating the effects of surgical stress on human tumor tissues is crucial for determining the full effect of this stressor on tumorigenesis and for devising effective interventions. Our results indicate that surgical stress promotes tumor growth by activating ADRB on tumor cells. Biologically, the acceleration in tumor growth is associated with increased angiogenesis and increased VEGF production. This premise is supported by our finding that even surgery at an extraperitoneal site (i.e., mastectomy) promoted i.p. tumor growth (Fig. 1). Our findings related to the efficacy of a β-blocker in abrogating surgically induced tumor growth suggest that such an approach may hold utility for cancer patients undergoing surgery (Fig. 2). Our findings about activation of angiogenesis by surgical stress are consistent with work from our and other groups showing the effects of stress on angiogenic factors (Figs. 3 and 4; refs. 24, 31).
Although we focused on sympathetic influence and angiogenesis in the current study, it is possible that other mechanisms might be operative as well in mediating the effects of stress. Therefore, we analyzed several cytokines and chemokines in serum following surgery (Fig. 5). Indeed, several cytokines were elevated, which might also be involved in the effects of surgical stress on tumor growth (32–34).
Results from the present study further expand our understanding of mechanisms by which surgical stress promotes tumor growth. Previous studies have shown decrements in immune function (e.g., suppressed natural killer cell response) following surgery that creates a permissive environment for tumor growth (29, 30). Our results using a partially immunodeficient model suggest direct pathways by which surgical stress can modulate the tumor microenvironment. It is possible that immune compromise along with direct effects of surgical stress on tumor cells may result in even greater promotion of tumor growth in immune competent models.
In summary, increased angiogenic processes induced by surgical stress promote tumor growth in vivo. These effects can be effectively blocked by a β-blocker such as propranolol. These findings could have significant implications for the perioperative management of cancer patients.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Grant support: National Cancer Institute T32 Training Grant CA101642, NIH grants CA110793 and CA109298, Ovarian Cancer Research Fund Program Project Development Grant, The University of Texas M. D. Anderson Cancer Center Ovarian Cancer Specialized Program of Research Excellence grant P50 CA083639, Gynecologic Cancer Foundation, Marcus Foundation, and Betty Ann Asche Murray Distinguished Professorship.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Acknowledgments
We thank Dr. Robert Langley for helpful discussions and Donna Reynolds for assistance with immunohistochemistry.