Abstract
Purpose: CD70, a member of the TNF ligand superfamily, has been shown frequently overexpressed in clear cell renal cell carcinoma (ccRCC). The mechanisms of CD70′s upregulation and its role in ccRCC are unknown.
Experimental Design: CD70 expression was immunohistochemically analyzed in 667 RCCs and RCC metastases. Von Hippel–Lindau gene (VHL) mutations, expression patterns of VHL protein (pVHL), hypoxia-inducible factor (HIF) α, and several HIF targets were studied in tissues and cell lines and correlated with CD70 overexpression. Gene promoter analysis was performed to confirm CD70 as HIF target gene. Consecutive tissue sections were immunostained to reveal the relation between CD70-expressing RCCs and tumor-infiltrating lymphocytes positive for the CD70 receptor (CD27). CD70-mediated release of soluble CD27 in RCC was assessed by coculture experiments and sera analysis of patients with RCC.
Results: Elevated CD70 expression was seen in 80% of primary tumors and metastases of ccRCC and correlated with dysregulation of the pVHL/HIF pathway. In vitro analyses demonstrated that CD70 upregulation is driven by HIF. Furthermore, CD27+ lymphocytes preferentially infiltrate CD70-expressing ccRCCs. CD70-dependent release of soluble CD27 in cocultures may explain the high CD27 levels observed in sera of patients with CD70-expressing ccRCC. The combination of lymphocyte infiltration and CD70 expression in RCC was associated with worse patient outcome.
Conclusion: Our findings demonstrate that in ccRCC, CD70 expression is regulated by HIF as a consequence of pVHL inactivation. Increased serum levels of CD27 suggest the existence of CD70-expressing ccRCC, thus representing a potential serum marker for patients suffering from this disease. Clin Cancer Res; 21(4); 889–98. ©2015 AACR.
This article is featured in Highlights of This Issue, p. 663
The deregulation of the von Hippel–Lindau protein (pVHL)/hypoxia-inducible factor (HIF) axis is a hallmark of clear cell renal cell carcinoma (ccRCC). Consequently, protein products of HIF-regulated genes are considered potential therapeutic targets of this tumor type. We have found that in ccRCC, abundant and frequent CD70 expression is directly driven by HIF. Furthermore, we observed a close relationship between CD70 overexpression and CD70 receptor (CD27)+ infiltrating lymphocytes, which seems to be linked to a more aggressive biologic behavior. Coculturing of pVHL-negative and pVHL-reexpressing RCC cell lines with peripheral blood mononuclear cells demonstrated significant increase of soluble CD27 levels in the presence of CD70. This may be the cause for the high soluble CD27 levels observed in the sera of patients with CD70-expressing ccRCC infiltrated by CD27+ lymphocytes, thus suggesting soluble CD27 as a diagnostic tool for ccRCC patient monitoring.
Introduction
About 70% of clear cell renal cell carcinoma RCC (ccRCC), the most common RCC subtype, harbor mutations in the von Hippel–Lindau gene (VHL; ref. 1). Its gene product, pVHL, acts as an adaptor protein for transcriptional regulation, the extracellular matrix, and the microtubule cytoskeleton (2). The most prominent function of pVHL is its role to target hypoxia-inducible factor (HIF) α for ubiquitin-mediated proteolysis. The loss of function of pVHL stabilizes HIFα and leads to the activation of a series of genes, which encode proteins involved in angiogenesis, metabolism, and cell-cycle regulation. Among them are cell surface proteins, including vascular endothelial growth factor and platelet-derived growth factor, which represent important therapy targets for patients with ccRCC (3). However, these treatments have significant toxic side effects and response is only seen in a subgroup of patients. To improve diagnosis, prognosis, and treatment of patients with RCC, additional biomarkers, in particular cell surface proteins, would be of utmost importance.
CD70 is a member of the TNF ligand superfamily and caught our interest because it was one of the ccRCC surface proteins we previously identified by cell surface capturing technology (4). Under normal conditions, CD70 expression is tightly regulated and restricted to a small subset of antigen-stimulated B and T lymphocytes and mature dendritic cells (5, 6). The interaction of CD70 with its receptor CD27 promotes expansion and differentiation of memory and effector T cells as well as B-cell expansion and plasma cell differentiation (7, 8). Altered CD70 expression was found in hematologic malignancies and also in solid tumors, such as nasopharyngeal carcinoma, thymic carcinoma, and brain tumors (9). The most frequent expression was reported in RCC (10–13), but the mechanism that leads to the upregulation of CD70 in this tumor type is still unclear. Interestingly, CD70 expression is less prevalent in VHL unrelated RCC subtypes. Studies elucidating the consequences of the constitutive expression of CD70 and its interaction with CD27 in ccRCC are rare and controversial. Although some authors hypothesized that CD70 is involved in an immune escape mechanism of RCC by mediating apoptosis in lymphocytes (14), others suggested that CD70 rather triggers a phenotypic conversion of CD27+ tumor-infiltrating lymphocytes (TIL) into a more differentiated state (15).
Here, we studied the impact of pVHL and HIFα on CD70 expression as well as the role of CD27-expressing TILs in ccRCC by (i) analyzing CD70 expression patterns in a large cohort of RCC tissues, (ii) investigating the interrelation of CD70 expression and the pVHL-HIFα axis by VHL mutation analysis and in vitro experiments, (iii) determining CD27 mRNA and protein expression in TILs and its correlation with the CD70 expression status, and (iv) examining the concentration of soluble CD27 (sCD27) in cocultures and sera of patients with CD70-positive and -negative tumors.
Materials and Methods
Patient material
For immunohistochemical (IHC) analyses, tissue microarrays (TMA) with 252 ccRCCs (4) and 54 ccRCC brain metastases (16, 17) from the Institute of Surgical Pathology, University Hospital of Zurich, Switzerland, and a TMA comprising 348 papillary RCCs (18) from the University of Erlangen, Erlangen, Germany, were used. Each TMA was histologically reviewed by one pathologist (H. Moch and A. Hartmann, respectively). Clinicopathological parameters of the first TMA have already been described (19). This study was approved by the local ethics commission (reference no. StV38-2005). Human blood samples of 54 patients, who underwent full or partial nephrectomy as part of their standard treatment at the Department of Urology, University Hospital Zurich (Zurich, Switzerland), were collected on the day of surgery following informed consent. Serum was processed according to a standardized protocol. Sera of 17 healthy persons served as control. Formalin-fixed, paraffin-embedded tissue samples from these patients were also available. This study was approved by the local ethics commission (KEK-ZH no. 2011-0072/4).
IHC
TMA and whole tumor sections (2.5 μm) were IHC-stained according to Ventana automat protocols (Ventana Medical System). The antibodies applied for detection are listed in Supplementary Table S1. The staining intensities were classified as absent, moderate, and strong. Lymphocyte infiltration was analyzed using hematoxylin and eosin-stained large sections and was scored as absent, sparse to loose and dense infiltrate. For detailed analysis, TMAs were scanned using the NanoZoomer Digital Slide Scanner (Hamamatsu Photonics K.K.).
VHL mutation analysis
Gene expression analysis
Gene expression data of 116 RCCs and six normal kidneys were obtained from a recently published microarray study (20). Microarray data are available in Gene Expression Omnibus under GSE19949.
Cell lines
The RCC-derived cell lines ACHN, A498, A704, Caki-1, Caki-2, HK-2, KC12, 769P, 786-O and the human embryonic kidney cell line HEK-293 were supplied by ATCC. RCC4, SLR21 to SLR26 were kindly provided by W. Krek (ETH Zurich, Switzerland) and W.J. Storkus (University of Pittsburgh, Pittsburgh, PA), respectively. Stable transfectants of 786-O and A498 reexpressing pVHL and empty vector controls were previously generated (21, 22). Cell lines were grown under conditions recommended by ATCC and authenticated by short tandem repeat profiling by Identicell (Aarhus University, Aarhus, Denmark). For immune staining, cell line pellets were formalin fixed and paraffin embedded as recently described (23). For hypoxia experiments, cells were incubated under low-oxygen conditions (0.5%) in a hypoxic workstation (Invivo2 400; Ruskinn Technology) for up to 24 hours.
Immunoblot analysis
Preparation of cell lysates, determination of protein concentration, and Western blotting were performed as described (4). Antibodies used are listed in Supplementary Table S1. For removal of N-glycans, PNGase F (New England Biolabs) was used.
Transient transfections
For RNAi experiments, cells were transfected with 5 nmol/L siRNA (HIF1α, HIF2α, and scrambled) as described (24). For silencing of CD70, Hs_TNFSF7_1;_2;_3, and Hs_CD70_1 (00748699, SI00748706, SI00748713, SI04277182, Qiagen) were used.
To study the effects of HIFα overexpression, RCC cells were transfected with 5 μg HIF1α, HIF2α, or HIF2α DNA-binding mutant (25) in pcDNA3.1 using XtremeGene HP (Roche) and grown for 24 hours in a six-well format. The mutant HIF2α construct was kindly provided by P. Ratcliffe (University of Oxford, Oxford, United Kingdom).
Quantitative reverse transcription-PCR
RNA isolation, cDNA synthesis, and quantitative reverse transcription-PCR (qPCR) were performed as described (4). TaqMan Gene Assays (Applied Biosystems) for CA-IX (Hs00154208_m1), CD70 (Hs00174297_m1), CD27 (Hs00386811_m1), GLUT1 (Hs00197884_m1), HIF1α (Hs00936376_m1), HIF2α (Hs01026146_m1), OCT4 (Hs04260367_gH), PDK1 (Hs01561850_m1), PHD3 (Hs00222966_m1), PPIA (Hs99999904_m1), and VHL (Hs00184451_m1) were used.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed as described (5). Fragments of 100-600 bp of cellular DNA were incubated with a HIFα-antibody/magnetic bead (Dynabeads Protein G; Invitrogen) mixture. SYBR Green real-time PCR was performed to quantify the differential binding of HIFα to the hypoxia-responsive elements (HRE) of CD70. The antibody and primers used for the ChIP experiments are listed in Supplementary Tables S1 and S3.
Luciferase gene reporter assay
The nucleotides between −2,000 and +700 were defined as the putative promoter region of CD70 (Chr 19p13; Gene-ID: 970). The promoter region was synthesized from GenScript and cloned into firefly luciferase reporter plasmid pGL4.10 (Promega). The positive control P2P contains the P2P HREs of the human PHD2 promoter (26) and were provided by R. Wenger (University of Zurich). Luciferase activity was measured 24 hours after transfection of A498 empty vector and A498 reexpressing pVHL with 1 μg of reporter plasmids along with 200 ng of pGL4.74 Renilla luciferase reporter plasmid in 24-well plates. HIFα overexpression was performed 48 hours before measurement. Relative luciferase units were determined according to the manufacturer's instructions (Dual luciferase reporter assay system, Promega).
Determination of sCD27 in cocultures and sera of patients with RCC
The RCC cells were seeded into six wells that the number of cells reached 2.7 × 105/well 48 hours later. For siRNA experiments, cell transfection was performed 24 hours after seeding. Peripheral blood mononuclear cells (PBMC) of a healthy individual were isolated by Ficoll (GE Healthcare) density centrifugation. Forty-eight hours after seeding and repeated washing, 2.7 × 106 PBMCs were added in 2 mL RPMI-1640 containing 10% FCS. The cells were cocultured for 2 additional days. PBMCs and RCC cells were maintained in monoculture in parallel. After 2 days, supernatants were used to analyze sCD27 concentration by sandwich ELISA (M1960, Sanquin) according to the manufacturer's instructions. The different experimental approaches were performed using PBMCs of different healthy individuals (donor A-C). The sCD27 ELISA was also used for testing sera of patients with RCC and healthy individuals.
Statistical and computational analyses
The Pearson χ2 test, Fisher exact test, Student t test, and survival analysis were performed using SPSS Statistics 21 (IBM). Correlation analysis (Pearson R) was performed using GraphPad Prism 5 (GraphPad Software).
Results
CD70 is frequently expressed in ccRCC and ccRCC metastasis
Gene expression analysis of 116 RCC tissues (20) revealed that CD70 was upregulated in primary and metastatic ccRCC (Fig. 1A). Using TMAs containing a total of 667 RCCs, we observed high CD70 protein expression in 78% of ccRCCs, but in only 32% of papillary RCC. Oncocytomas, chromophobe RCCs, and normal kidneys were negative (Fig. 1B and C). Furthermore, 54 ccRCC brain metastases showed a similar CD70 expression frequency as observed in primary tumors. Notably, the strong CD70 expression seen in nine primary ccRCC remained constant in their corresponding brain metastasis (Fig. 1D).
CD70 expression pattern in RCC. A, CD70 gene expression in 116 RCCs and six normal kidney tissues. T tests were performed. B, representative pictures of anti–CD70-immunostained ccRCCs and papillary RCCs (×40 magnification, bars: 20 μm). C, histogram representing CD70 expression intensities in RCC and normal kidney tissue. The Pearson χ2 test or Fisher exact test was performed. D, representative pictures of anti–CD70-immunostained localized ccRCC and corresponding brain metastasis (n = 8,×20 magnification, bars, 50 μm). pap, papillary; chromo, chromophobe; met, metastasis; ***, P < 0.0001; **, P < 0.005; *, P < 0.05; ns, not significant.
CD70 expression pattern in RCC. A, CD70 gene expression in 116 RCCs and six normal kidney tissues. T tests were performed. B, representative pictures of anti–CD70-immunostained ccRCCs and papillary RCCs (×40 magnification, bars: 20 μm). C, histogram representing CD70 expression intensities in RCC and normal kidney tissue. The Pearson χ2 test or Fisher exact test was performed. D, representative pictures of anti–CD70-immunostained localized ccRCC and corresponding brain metastasis (n = 8,×20 magnification, bars, 50 μm). pap, papillary; chromo, chromophobe; met, metastasis; ***, P < 0.0001; **, P < 0.005; *, P < 0.05; ns, not significant.
CD70 expression and VHL mutation status in ccRCC tissues and cell lines
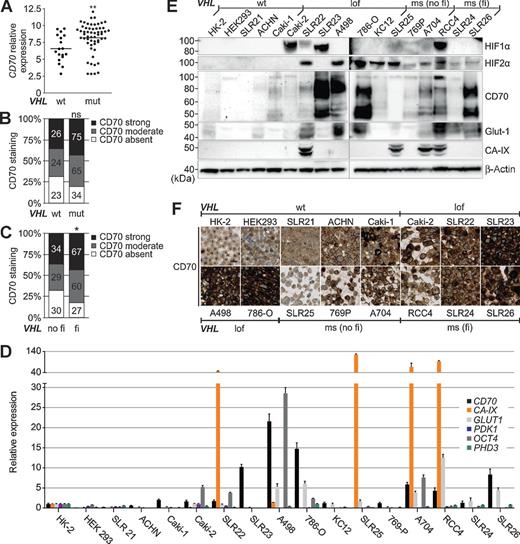
Many VHL mutations are thought to inactivate pVHL's functional integrity in ccRCC. To test whether VHL mutations affect CD70 expression, we correlated the mRNA and protein expression patterns of CD70 with VHL mutation data in a series of ccRCCs, which were recently published by our group (1). Although VHL wild-type (wt) ccRCC may also have lost pVHL activity due to hypermethylation of its gene promoter, we found an association of CD70 expression and mutated VHL (Fig. 2A and B). CD70 expression was even more frequent in tumors with VHL mutations, which predict impaired function of pVHL compared with tumors with VHL wt and VHL mutations not affecting the protein function (Fig. 2C). We confirmed these findings in RCC cell lines with different VHL and HIF1α mutation states (ref. 27; Supplementary table S2). CD70 mRNA and protein levels were generally higher in RCC cell lines harboring VHL loss-of-function mutations (Caki-2, SLR22, SLR23, A498, 786-O, and KC12) and VHL missense mutations predicted to affect the pVHL function (RCC4, SLR24, and SLR26). RCC cell lines with VHL missense mutations having no functional impact on the protein (SLR25, 769P, and A704) and those with wt VHL (SLR21, ACHN, Caki-1, HK-2, and HEK293) showed no or only weak CD70 expression (Fig. 2D–F). CD70 expression was mainly observed in cell lines that either were HIF1α-, HIF2α- or HIF1α- and HIF2α‐positive. In most of the cell lines that express CD70, Glut1 and CA-IX were also present (Fig. 2E). This was also true on mRNA level, the CD70 expression correlated with GLUT1 and CA-IX, but was not associated with other HIF target genes (PDK1, OCT4, and PHD3; Fig. 2D). The multiple banding pattern of CD70 in RCC seen on Western blot analyses was also previously described (13, 28) and results largely from N-linked glycosylation (Supplementary Fig. S1).
Association of CD70 with the VHL mutation status. A, CD70 gene expression in ccRCC tissue with mutated or wt VHL. **, P < 0.001 by t test. B, histogram representing CD70 expression intensities in ccRCCs with mutated or wt VHL. ns, not significant by Pearson χ2 test. C, CD70 expression intensities assigned to ccRCC tissue with function of pVHL predicted to be impaired or not impaired. In silico analysis was done with SDM. *, P < 0.05 by Pearson χ2 test. D, mRNA levels of CD70, the HIF1α-dependent genes CA-IX and PDK1, the HIF2α-dependent genes GLUT1, OCT4, and PHD3; (E) Western blot analysis of HIF1α, HIF2α, CD70, CA-IX, and Glut1; and (F) cell microarray stained for CD70 in different RCC cell lines (×20 magnification; bar, 20 μm). The VHL mutation status is indicated as wt, loss of function (lof), missense (ms) with (fi) and without (no fi) functional impact on pVHL.
Association of CD70 with the VHL mutation status. A, CD70 gene expression in ccRCC tissue with mutated or wt VHL. **, P < 0.001 by t test. B, histogram representing CD70 expression intensities in ccRCCs with mutated or wt VHL. ns, not significant by Pearson χ2 test. C, CD70 expression intensities assigned to ccRCC tissue with function of pVHL predicted to be impaired or not impaired. In silico analysis was done with SDM. *, P < 0.05 by Pearson χ2 test. D, mRNA levels of CD70, the HIF1α-dependent genes CA-IX and PDK1, the HIF2α-dependent genes GLUT1, OCT4, and PHD3; (E) Western blot analysis of HIF1α, HIF2α, CD70, CA-IX, and Glut1; and (F) cell microarray stained for CD70 in different RCC cell lines (×20 magnification; bar, 20 μm). The VHL mutation status is indicated as wt, loss of function (lof), missense (ms) with (fi) and without (no fi) functional impact on pVHL.
CD70 expression is dependent of the pVHL/HIFα axis in RCC tissue and cell lines
To evaluate the impact of pVHL and HIF on CD70 expression in RCC, we first performed qPCR (Fig. 3A) and Western blot analyses (Fig. 3B) of pVHL-deficient RCC cell lines 786-O and A498 and their stable transfectants reexpressing pVHL. Reconstituted pVHL lead to a decrease of the CD70 expression and the HIF targets CA-IX, GLUT1, OCT4, and PHD3. TMA analyses demonstrated a significant association of CD70 expression and pVHL/HIF deregulation. CD70 expression was directly correlated with the expression of HIF1α (P = 0.0136, Pearson R = 0.1359) and HIF2α (P < 0.0001, Pearson R = 0.2833) as well as with the HIF targets CA-IX (P < 0.0001, Pearson R = 0.4267), Glut1 (P < 0.0001, Pearson R = 0.4093), and CD10 (ref. 4; P < 0.0001, Pearson R = 0.3901). An inverse correlation was seen with pVHL (P = 0.0144, Pearson R = −0.1398) and E-Cadherin (P = 0.0485, Pearson R = −0.1121). CD70 expression alone was not associated with grade, stage, and survival in ccRCC (data not shown). Silencing of HIF2α in 786-O and A498 exclusively expressing HIF2α (but not HIF1α) lead to a decrease of CD70 mRNA and protein expression (Fig. 3C–F). In SLR22, CD70 downregulation was achieved only when both HIF1α and HIF2α were silenced (Supplementary Fig. S2A). Following transient overexpression of HIF1α and HIF2α in 786-O and A498, an increase of CD70 mRNA and protein levels was observed (Fig. 3G and H). Even in the pVHL-defective RCC cell lines SLR22 and SLR24 with low endogenous CD70 levels, upregulation of the latter could be induced by overexpression of HIF2α (Supplementary Fig. S2B and S2C). In pVHL-negative and -positive 786-O and A498 cells, CD70 expression was continuously increasing under hypoxia within the first 12 hours (Fig. 3H and I).
CD70 expression is dependent on the pVHL/HIF axis. A, mRNA levels of VHL, CD70, CA-IX, GLUT1, OCT4, and PHD3 in 786-O and A498 with reconstituted pVHL relative to empty vector obtained by qPCR. B, Western blot analysis of HIF2α, CD70, and pVHL in 786-O and A498 with reconstituted pVHL or empty vector. C, mRNA levels of CD70, CA-IX, GLUT1, OCT4, PHD3, and HIF2α relative to control siRNA (siScr) obtained by qPCR and (D) Western blot analysis of HIF2α and CD70 in 786-O, and (E) A498 after silencing of HIF2α for 48 and 72 hours. F, mRNA levels of CD70, CA-IX, HIF1α, and HIF2α relative to empty vector control obtained by qPCR and (G) Western blot analysis of HIF1α, HIF2α, CA-IX, and CD70 in 786-O after transient overexpression of HIF1α and HIF2α. H, Western blot analysis of HIF2α and CD70 in 786-O and (I) A498 with and without reconstituted pVHL incubated at 0.5% oxygen for 0, 2, 4, 6, 12, and 24 hours (h). Error bars, SD (n = 3).
CD70 expression is dependent on the pVHL/HIF axis. A, mRNA levels of VHL, CD70, CA-IX, GLUT1, OCT4, and PHD3 in 786-O and A498 with reconstituted pVHL relative to empty vector obtained by qPCR. B, Western blot analysis of HIF2α, CD70, and pVHL in 786-O and A498 with reconstituted pVHL or empty vector. C, mRNA levels of CD70, CA-IX, GLUT1, OCT4, PHD3, and HIF2α relative to control siRNA (siScr) obtained by qPCR and (D) Western blot analysis of HIF2α and CD70 in 786-O, and (E) A498 after silencing of HIF2α for 48 and 72 hours. F, mRNA levels of CD70, CA-IX, HIF1α, and HIF2α relative to empty vector control obtained by qPCR and (G) Western blot analysis of HIF1α, HIF2α, CA-IX, and CD70 in 786-O after transient overexpression of HIF1α and HIF2α. H, Western blot analysis of HIF2α and CD70 in 786-O and (I) A498 with and without reconstituted pVHL incubated at 0.5% oxygen for 0, 2, 4, 6, 12, and 24 hours (h). Error bars, SD (n = 3).
CD70 expression is directly regulated by HIF
To elucidate whether CD70 represents a potential HIF target, we investigated HIF's ability to bind to the eight potential HREs residing in 2.7 kb of the putative promoter of CD70 (Fig. 4A) by ChIP using HIF2α-, HIF1α-, and the corresponding isotype control- antibodies (Fig. 4B and C). The reconstitution of pVHL in 786-O, A498, and RCC4 leads to a reduction of the direct binding of HIF2α and HIF1α to HRE3, 4, and 8 in the CD70 promoter. No binding was observed for the negative control gene KCNJ5. CD10 HRE2 served as positive control for HIF1α binding (4).
CD70 promoter binding and gene expression by HIFα. A, schematic representation of the 2.7 kb CD70 promoter with the location of six HREs and two reverse HREs (rHRE). B, binding of HIF2α and (C) HIF1α at the CD70 promoter relative to its isotype controls analyzed by ChIP. HRE1-8 indicates in silico identified HREs in the CD70 promoter; KCNJ5 served as negative and CD10 HRE2 as positive control for HIF1α. Results are shown as ratios of the amplicons detected in 786-O, A498, or RCC4 pVHL-positive and negative relative to HRE1. D, luciferase induction of the 2.7 kb promoter region of CD70 in A498 empty vector relative to A498 reconstituted with pVHL and (E) after overexpression of HIF2α or a DNA binding mutant of HIF2α in A498 relative to pcDNA3.1-transfected cells. P2P served as a positive control for the HIF activity. (vector = empty luciferase reporter plasmid pGL4.10). Error bars, SD (n = 3).
CD70 promoter binding and gene expression by HIFα. A, schematic representation of the 2.7 kb CD70 promoter with the location of six HREs and two reverse HREs (rHRE). B, binding of HIF2α and (C) HIF1α at the CD70 promoter relative to its isotype controls analyzed by ChIP. HRE1-8 indicates in silico identified HREs in the CD70 promoter; KCNJ5 served as negative and CD10 HRE2 as positive control for HIF1α. Results are shown as ratios of the amplicons detected in 786-O, A498, or RCC4 pVHL-positive and negative relative to HRE1. D, luciferase induction of the 2.7 kb promoter region of CD70 in A498 empty vector relative to A498 reconstituted with pVHL and (E) after overexpression of HIF2α or a DNA binding mutant of HIF2α in A498 relative to pcDNA3.1-transfected cells. P2P served as a positive control for the HIF activity. (vector = empty luciferase reporter plasmid pGL4.10). Error bars, SD (n = 3).
To confirm this finding, we performed luciferase reporter gene assays. For this purpose, the DNA fragment containing the 5′-region of CD70 (−2000/+700) was inserted upstream of the luciferase gene. Compared with pVHL reexpressing A498 cells, the luciferase activity of the CD70 reporter gene construct was increased in A498 empty vector cells to the same extent as for the HIF target gene PHD2 promoter construct (P2P; Fig. 4D).The same effect was seen by overexpression of HIF2α in A498, but not by overexpression of its DNA-binding mutant (ref. 25; Fig. 4E), which is unable to induce the expression of the CD70 protein product (Supplementary Fig. S2D and S2E).
Preferential infiltration of CD27+ lymphocytes in CD70-expressing tumors
As the role of CD70–CD27 interaction in ccRCC is unknown, we next asked whether there was an association between CD70-expressing tumor cells and CD27+ TILs. qPCR analysis of 47 ccRCCs selected from the TMA, which was immunostained against CD70, showed higher CD27 mRNA expression in tumors with strong CD70 staining compared with tumors with lower CD70 expression (Fig. 5A). CD27 and CD70 gene expression obtained from a recent DNA-microarray analysis (20) confirmed this finding (Fig. 5B). To validate the gene expression data on protein level, we investigated CD70 and CD27 expression patterns on large consecutive tissue sections of 41 ccRCC infiltrated by lymphocytes. We observed that 37 ccRCCs (90%) contained also CD27+ lymphocytes (Fig. 5C). Interestingly, correlations between TMA expression data and clinicopathological data showed that patients with CD70-negative ccRCCs and no lymphocyte infiltration had a better survival compared with those with CD70 strongly positive and lymphocyte-infiltrated ccRCCs (P < 0.05; Fig. 5D). The latter tumors were significantly associated with a high Fuhrman grade (P < 0.0001) but not with late tumor stage (Supplementary table S4). The strong correlation between CD27 and the subunits of the T-cell marker CD3 indicates that the CD27-stained lymphocytes were T cells (Supplementary Fig. S3).
Correlation between CD70 expression and CD27+ TILs. A, CD27 mRNA levels obtained by qPCR separated into ccRCCs showing no, moderate, or strong CD70 tumor staining on TMA. B, CD70 plotted against CD27 mRNA levels of the gene expression data. Pearson R correlation analyses are depicted. (met, metastasis). C, representative pictures of anti-CD70 and anti–CD27-immunostained ccRCCs of consecutive slides (×40 magnification; bar, 100 μm). D, Kaplan–Meier plot of the overall survival of ccRCC patients defined by the CD70 expression accompanied by lymphocyte infiltration. A log-rank test was performed.
Correlation between CD70 expression and CD27+ TILs. A, CD27 mRNA levels obtained by qPCR separated into ccRCCs showing no, moderate, or strong CD70 tumor staining on TMA. B, CD70 plotted against CD27 mRNA levels of the gene expression data. Pearson R correlation analyses are depicted. (met, metastasis). C, representative pictures of anti-CD70 and anti–CD27-immunostained ccRCCs of consecutive slides (×40 magnification; bar, 100 μm). D, Kaplan–Meier plot of the overall survival of ccRCC patients defined by the CD70 expression accompanied by lymphocyte infiltration. A log-rank test was performed.
PBMCs trigger the release of sCD27 in a CD70-dependent manner
CD27 is known to be cleaved by a protease into its soluble form sCD27 on activated T cells (29, 30). It was also shown that CD70-expressing glioma cells enhance the release of sCD27 from PBMCs (28). To test whether CD27 can also be cleaved after interaction with CD70 in RCC, we cocultured RCC cells with freshly isolated PBMCs and measured sCD27 in the supernatant by ELISA (Fig. 6A). Supernatants of PBMC and RCC cell monocultures contained basic levels of sCD27 after 2 days. PBMC cocultures with the CD70-expressing cell lines 786-O, A498, and SLR23 significantly increased the sCD27 concentration (2.7-fold, 1.9-fold, and 2.3-fold, respectively). This increase correlated with the PBMC/RCC cells ratio (Supplementary Fig. S4A). Cocultures with CD70 low-expressing cell lines Caki-2, SLR22, SLR24, and SLR25 had only slightly elevated sCD27 concentration levels compared with PBMC monocultures. Both CD70 silencing (Supplementary Fig. S4B and S4C) and reconstitution of pVHL in 786-O and A498 cells caused a reduction of sCD27 levels (Fig. 6A).
Release of sCD27 via CD70 in vitro and high serum levels of sCD27 in CD70-expressing RCC patients. A, concentrations of sCD27 in supernatants of PBMC and RCC cell line cocultures or monocultures were determined by ELISA. In 786-O and A498, CD70 expression was silenced by siRNA (siCD70#1–4). siScr, control siRNA. Donor A–C, PBMCs from different healthy individuals. B, sCD27 serum concentrations of patients with RCC and healthy controls. **, P < 0.005 by the t test. C, representative pictures of anti-CD70 and anti–CD27-immunostained ccRCCs of consecutive slides of large sections (×40 magnification; bar, 100 μm). The existence of CD70 expression and CD27+ infiltrations (CD70/CD27) of the tumor tissues was indicated as absent (−) or present (+). D, a model showing the biologic and clinical relevance of CD70 and CD27 in ccRCC.
Release of sCD27 via CD70 in vitro and high serum levels of sCD27 in CD70-expressing RCC patients. A, concentrations of sCD27 in supernatants of PBMC and RCC cell line cocultures or monocultures were determined by ELISA. In 786-O and A498, CD70 expression was silenced by siRNA (siCD70#1–4). siScr, control siRNA. Donor A–C, PBMCs from different healthy individuals. B, sCD27 serum concentrations of patients with RCC and healthy controls. **, P < 0.005 by the t test. C, representative pictures of anti-CD70 and anti–CD27-immunostained ccRCCs of consecutive slides of large sections (×40 magnification; bar, 100 μm). The existence of CD70 expression and CD27+ infiltrations (CD70/CD27) of the tumor tissues was indicated as absent (−) or present (+). D, a model showing the biologic and clinical relevance of CD70 and CD27 in ccRCC.
Elevated sCD27 levels are detectable in sera of patients with CD70-expressing RCC
To investigate whether our in vitro results were conferrable to patients, we measured sCD27 concentration in sera of ccRCC and papillary RCC patients and healthy individuals (Fig. 6B). sCD27 levels were compared with the expression status of CD27 and CD70 on large tissue sections of the corresponding patients (Fig. 6C). sCD27 serum levels of patients with CD70-expressing tumor cells and CD27+ TILs were significantly higher compared with those of healthy individuals and RCCs of patients that either had CD70-positive tumor cells but no CD27+ TILs or had CD27+ TILs and CD70-negative tumor cells or were negative for both.
Notably, ELISA analysis of CD70 revealed that CD70 protein expression levels in 31 RCCs were not reflected in the sera of these patients as most of the CD70 levels were below the detection limit (data not shown).
Discussion
In this study, we demonstrated that the high and frequent CD70 expression in ccRCC is driven by HIF as a consequence of pVHL loss of function. We further observed a strong association between CD70-expressing tumor cells and the presence of CD27+ T cells in TILs. The ability of CD70 to trigger the release of sCD27 on PBMCs in vitro and the increased levels of sCD27 in the sera of patients with CD70-expressing tumors suggest that sCD27 is a putative serum marker for CD70-positive ccRCC patients.
CD70 expression is dependent on the RCC subtype. IHC TMA staining of more than 600 RCCs showed abnormal CD70 expression in 78% of ccRCCs, which was consistent with previously described frequencies (11, 12). Our comprehensive CD70 analysis of 348 papillary RCCs revealed 11% strongly and 22% moderately expressing tumors, which was lower compared with the frequencies observed in recently published smaller cohorts (10, 12, 13). No expression was seen in 21 oncocytomas and 13 chromophobe RCCs suggesting a negligible role of CD70 in these tumor subtypes.
We show that the abnormal upregulation of CD70 in ccRCC is closely related to the deregulation of the pVHL/HIF axis. CD70 expression patterns in RCC tissue were significantly correlated with those seen for HIFα and the HIF targets CA-IX, Glut1, and CD10. The analysis of the mutation status of VHL in 363 ccRCCs and 17 RCC cell lines further supported the association of CD70 expression with VHL sequence alterations. High CD70 expression levels were significantly linked to VHL mutations that very likely abrogate pVHL's function. Inactivation of VHL by hypermethylation of its promoter (31) and missense mutations with no or only minor effects on pVHL's ability to bind HIFα (1) may explain the presence or absence of CD70 in several VHL wt and mutated tumors, respectively. In pVHL-affected cell lines, the expression pattern of CD70 was comparable with that of HIF1α, HIF2α as well as HIF targets Glut1 and CA-IX. Our results suggest that CD70 is driven by both HIF1α and HIF2α. Some pVHL-deficient ccRCC cell lines exhibited elevated HIFα although CD70 was absent. They may resemble those 20% (34/174) of CD70-negative ccRCCs, which had VHL mutations with functional impact on pVHL, presumably causing HIFα stabilization. Epigenetic regulation of HIF-binding sites can probably influence the transcriptional response to hypoxia (32). Aberrant DNA methylation of the two CpG islands (−993 to −323 bp relative to transcription start site; ref. 33), which overlap the region with the putative HREs in the CD70 promoter, may thus prevent HIF from binding and may explain why CD70 is not or only weakly expressed in the presence of HIF in those tumors.
In vitro experiments, which included pVHL reconstitution, HIFα silencing and overexpression, hypoxia experiments, ChIP and luciferase assays confirmed that CD70 expression is dependent on pVHL's functional integrity and the transcriptional activation by HIF. The attenuation of CD70 protein expression after 12 hours under hypoxic conditions may be caused by oxygen-sensitive pathways that are HIF-independent (34).
It is obvious that in ccRCC, the mechanism of CD70′s upregulation is different to that reported in several T-cell affecting diseases, such as systemic lupus erythematosus and systemic sclerosis (35, 36) in which hypomethylation of the CD70 promoter contributes to CD70 overexpression. Using the same methylation-specific PCR protocol described by Yu and colleagues (33), no hypomethylation of the CD70 promoter was seen in ccRCC tissues, which strongly expressed CD70 (data not shown). This suggests that the transcriptional regulation of CD70 is rather driven by HIF and does not depend on promoter hypomethylation in this tumor subtype.
A strong relationship between HIFα activation, CD70 upregulation, and tumor formation was demonstrated in TRACK mice (transgenic model of cancer of the kidney), which specifically express a mutated, constitutively active form of HIF1α in kidney proximal tubule cells (37). Activated HIF1α was sufficient to promote phenotypic alterations characteristic of human VHL ccRCC, including upregulation of CA-IX, Glut1, and CD70, whereas a constitutively activated form of HIF2α was neither able to promote ccRCC nor to enhance the expression of CD70 (38). This stands in contrast with the findings of Gordan and colleagues (39) who showed that HIF2α rather than HIF1α mediates ccRCC progression. Our in vitro data indicate that CD70 can be activated by both HIFα isoforms. However, strong CD70 expression is preferentially seen in RCC cell lines in which only HIF2α is present suggesting that HIF2α is a key regulator for CD70 expression in progressing ccRCC.
In addition to the mechanisms for abnormal CD70 expression in ccRCC, we were also interested to investigate the role of the interaction with its receptor CD27, which is known to be expressed on the surface of memory B cells, naïve, and activated T cells and a subset of natural killer (NK) cells (7). Microarray gene expression data of ccRCC revealed a significant correlation between CD70 and CD27 expression. A detailed IHC analysis of large ccRCC tissue sections demonstrated that the lymphocyte infiltrates in CD70-expressing tumors were almost always accompanied by CD27+ TILs.
Neither we nor others have found a strong correlation between CD70 expression alone and tumor grade, stage, and patient survival (12, 13). As the loss of pVHL function is considered an early event in ccRCC (3), the subsequent upregulation of CD70 may also contribute to tumor formation. We observed that the frequency of strong CD70 expression in pVHL-deficient primary ccRCC is retained in metastatic lesions. It is therefore conceivable that the persistent expression of CD70 in tumors leads to an enhanced attraction and infiltration of CD27+ lymphocytes. Interestingly, ccRCC with strong CD70 expression and TILs had a higher nuclear differentiation grade and a significantly worse survival compared with CD70-negative tumors that were not infiltrated by lymphocytes. On the basis of this finding, we conclude that the interaction of CD70-expressing tumor cells and CD27+ TILs may play a role in tumor progression.
Different tumor-promoting mechanisms have been proposed in this context. sCD27 can induce proliferation in CD70-positive acute lymphoblastic leukemia and nasal NK/T-cell lymphoma (40, 41). It was therefore tempting to speculate that the interaction of CD27+ TILs with CD70 present on ccRCC cells has similar effects and accelerate tumor growth. Both sCD27 (42) and CD70 (8) contribute to T-cell activation and proliferation. However, we could not see any proliferative effects of recombinant sCD27 in response to CD70 in two RCC cell lines (data not shown). Second, although CD70-expressing ccRCC and glioma cells were able to provoke T-cell apoptosis in coculture experiments (14, 28, 43), the immune inhibitory properties of CD70 in vitro seem to be clearly overwhelmed by the immune activating properties observed in mouse glioma models in vivo (44). Finally, the chronic ligation of CD27 and CD70 may also lead to T-cell exhaustion, a kind of “immune escape.” It was shown that constitutive expression of CD70 on B cells in mice converted naïve T cells into effector memory cells. This culminated in the depletion of naïve T cells, which lead to lethal immunodeficiency (45). In keeping with this, Wang and colleagues observed less naïve and central memory T-cell subpopulations but more effector memory cells in ccRCC in contrast with peripheral blood lymphocytes and melanoma (15). They suggested that this phenotypic conversion of T cell is caused by high CD70 expression in ccRCC. These results together with our findings suggest that the coexistence of CD70 and CD27 in ccRCC fosters immune exhaustion effects by chronic costimulation.
As it was shown with glioma cells (28), coculturing of PBMCs with RCC cells resulted in a CD70-dependent increase of sCD27 in the supernatant. A similar effect may occur in CD70-expressing ccRCC tissue with CD27+ TILs because the levels of sCD27 in sera of those patients were significantly higher compared with patients with CD70- and CD27-negative tumors and healthy persons. Besides lymphoid malignancies (40, 41), ccRCC is the first solid tumor type in which increased serum levels of sCD27 have been observed. High serum concentration of sCD27 may thus represent a potential predictive marker in patients with ccRCC, which indirectly indicates the presence of CD70-expressing tumors.
In conclusion, we demonstrated that in pVHL-deficient ccRCC, CD70 is upregulated via HIF. A model illustrating our hypothesis is shown in Fig. 6D. CD70 and CD27 interaction seems to boost the release of sCD27 in sera of patients with ccRCC suggesting CD27 a potential prognostic and diagnostic marker that may also be used to predict the applicability of CD70-targeted therapies or CD27-targeted immunotherapies (46). At the moment, three novel anti-CD70 antibody–drug conjugates are under evaluation for metastatic ccRCC in clinical trial phase I (47), which have shown promising antitumor activity in CD70-expressing RCC mouse xenografts (48). One should, however, bear in mind that blocking of CD70–CD27 interaction by CD70-targeting drugs may impede de novo T-cell priming (49, 50), thus hampering patients' immune response capability.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors' Contributions
Conception and design: M. Ruf, H. Moch, P. Schraml
Development of methodology: M. Ruf, C. Mittmann, A.M. Nowicka, H. Moch
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): M. Ruf, C. Mittmann, A.M. Nowicka, A. Hartmann, T. Hermanns, C. Poyet, T. Sulser
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): M. Ruf, M. van den Broek, T. Sulser, H. Moch, P. Schraml
Writing, review, and/or revision of the manuscript: M. Ruf, A. Hartmann, T. Hermanns, M. van den Broek, T. Sulser, H. Moch, P. Schraml
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): M. Ruf, A. Hartmann, T. Hermanns, H. Moch
Study supervision: H. Moch, P. Schraml
Acknowledgments
The authors thank Susanne Dettwiler, Martina Storz, Adriana von Teichman, Sonja Brun-Schmid and André Fitsche (Institute of Surgical Pathology, University Hospital Zurich) for their excellent technical support; Alexandra Veloudios (Department of Urology, University Hospital Zurich) for collecting RCC patients sera; Markus Rechsteiner (Institute of Surgical Pathology, University Hospital Zurich) for supporting experimental setups; and Jannie Borst (Netherlands Cancer Institute, Amsterdam, the Netherlands) for valuable suggestions and for critically reading the manuscript.
Grant Support
This work was supported by the Swiss National Cancer Foundation (to H. Moch; 3238BO-10314) and the Zurich Cancer League (to H. Moch).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.