Abstract
Purpose: Both heat shock protein 90 (Hsp90) and checkpoint kinase 1 (Chk1) have emerged as novel therapeutic targets. We conducted a phase I study of irinotecan and the Hsp90 inhibitor 17AAG, which can also down-regulate Chk1, in patients with solid tumors.
Experimental Design: During the dose escalation phase, patients received i.v. irinotecan followed by 17AAG once weekly for 2 weeks in a 21-day cycle. At the maximum tolerated dose (MTD), additional patients were enrolled to undergo pre- and post-17AAG tumor biopsies for pharmacodynamic evaluation. The pharmacokinetics of irinotecan, 17AAG, and their metabolites were characterized. Tumor p53 status as determined by immunohistochemistry was correlated with antitumor activity.
Results: Twenty-seven patients with a variety of solid tumors were enrolled. Four patients developed dose-limiting toxicity at dose level 4 (100 mg/m2 irinotecan and 375 mg/m2 17AAG) including nausea, vomiting, diarrhea, and pulmonary embolism. The pharmacokinetics of 17AAG and its metabolite were not significantly affected by the coadministration of irinotecan, and vice versa. There was no partial response, although tumor shrinkage was observed in six patients. Five of 10 patients with p53-mutant tumor had stable disease as the best response compared with 2 of 6 patients with p53-wildtype tumor (P = 0.63). Evidence for Hsp90 inhibition by 17AAG, resulting in phospho-Chk1 loss, abrogation of the G2-M cell cycle checkpoint, and cell death could be shown in tumor biopsy samples obtained at the MTD.
Conclusions: The combination of irinotecan and 17AAG can be given to patients with acceptable toxicity. The recommended phase II dose of the combination is 100 mg/m2 irinotecan and 300 mg/m2 17AAG.
Heat shock protein 90 (Hsp90) and checkpoint kinase 1 (Chk1) are two novel targets for cancer therapy. Of interest to both areas of targeted therapeutics is the identification of Chk1 as an Hsp90 client protein. We embarked on this phase I study based on our preclinical work showing that 17AAG, the first Hsp90 inhibitor in clinical trial, could enhance the cytotoxicity of topoisomerase I poison, by depleting Chk1 and abrogating the cytoprotective G2-M cell cycle checkpoint.
We show in this combination study that 17AAG can be given at its full single-agent dose with acceptable toxicity. Although there was no partial response by response evaluation criteria in solid tumors, we observed minor tumor shrinkage in six patients treated by the combination. We obtained pre- and post-17AAG tumor biopsies to evaluate the pharmacodynamic effects of combined irinotecan and 17AAG. Evidence for Hsp90 inhibition by 17AAG, resulting in phospho-Chk1 loss, abrogation of the G2-M cell cycle checkpoint, and cell death could be shown in some tumor biopsy samples. To our knowledge, these pharmacodynamic studies showed for the first time that Chk1-mediated signaling can be disrupted by an Hsp90 inhibitor in human tumors, providing the proof-of-mechanism of this therapeutic approach and paving the way for future Hsp90/Chk1 inhibitor studies.
The molecular chaperone heat shock protein 90 (Hsp90) is essential for promoting the stability and functional maturation of a number of signaling proteins including Her2, Raf-1, Akt, C-Met, and HiF-1α (1). 17AAG inhibits the amino-terminus ATPase activity of Hsp90, resulting in the destabilization of its chaperoned “clients,” thereby allowing the simultaneous targeting of multiple oncogenic signaling pathways in tumors. Preclinical studies of 17AAG revealed promising antitumor activities in cell culture and xenograft models (2–4). Phase I studies of 17AAG showed acceptable toxicity and down-regulation of Hsp90 clients in normal and tumor tissues following treatment (5–9).
Preclinical data also showed that 17AAG sensitizes tumors to chemotherapy including nucleoside analogues (10, 11) and topoisomerase I poisons (12). One possible mechanism by which 17AAG enhances the cytotoxicity of chemotherapy is by depleting checkpoint kinase 1 (Chk1), a Hsp90 client kinase that is critical for the S and G2-M cell cycle checkpoints. We have shown that 17AAG selectively abrogates the G2-M checkpoint induced by a topoisomerase I poison and promotes apoptosis in colon cancer cells that lack p53. We therefore conducted a phase I study of 17AAG and irinotecan to determine the toxicity profile, maximum tolerated dose (MTD), pharmacokinetics, and pharmacodynamics of the combination.
Patients and Methods
Patient selection. Patients with histologically confirmed solid tumors refractory to standard treatment, or for which no standard therapy exists, were considered. Other eligibility criteria included: age ≥18 y; Karnofsky performance status ≥60 y; WBC count ≥3,000/mm3; absolute neutrophil count ≥1,500/mm3; platelet count ≥100,000/mm3; creatinine ≤1.5 mg/dL; total bilirubin ≤1.5 mg/dL; aspartate aminotransferase and alanine aminotransferase levels ≤3× upper limit of normal (or ≤5× upper limit of normal if there were known liver metastases); and normal serum potassium, magnesium, and calcium (or ionized calcium). Prior treatment with irinotecan but not 17AAG was allowed.
Patients were excluded if they were pregnant or lactating, had new untreated central nervous system metastases, primary brain tumor, HIV infection, severe egg allergy, or were taking potent CYP3A4 inducers or inhibitors. Because of supraventricular arrhythmias and pulmonary toxicity associated with 17AAG, patients at risk for cardiopulmonary toxicities were excluded, including those with a history of prior chest radiation, active ischemic heart disease or cardiac arrhythmias, left bundle branch block, heart failure, prolonged QTc, baseline diffusion-limited carbon monoxide ≤80%, baseline ejection fraction <50%, and debilitating pulmonary diseases.
Study design and treatment plan. This phase I study consisted of a dose escalation and an expansion phase. During dose escalation, three to six patients per dose level were treated with escalating doses of irinotecan and 17AAG from 85 and 220 mg/m2, respectively, up to the highest levels of 125 and 450 mg/m2, respectively, or until a dose-limiting toxicity (DLT) was observed in ≥2 of 3 or ≥2 of 6 patients. Irinotecan was given as a 30-min infusion followed by a 120-min infusion of 17AAG once weekly for two consecutive weeks in a 21-d cycle. To study possible pharmacokinetic interactions between the two agents, irinotecan alone was given on day 1 and 17AAG on day 2 during the second cycle (see Supplementary Fig. S1 for study design schema).
Toxicity was graded according to National Cancer Institute Common Toxicity Criteria Version 3.0. A DLT was defined as the occurrence of grade 4 hematologic toxicity, febrile neutropenia (absolute neutrophil count <1,000/mm3 and fever ≥38.5°C), ≥grade 3 nonhematologic toxicity, and any delay in treatment for >7 d. Patients received treatment at the same dose in the absence of DLT if, on the day of scheduled treatment, the absolute neutrophil count was ≥1,500/mm3 and platelet count ≥100,000/mm3; otherwise, therapy was held. A minimum of three patients were followed for at least one complete cycle of therapy before the dose was escalated to the next level. If a DLT was observed, the cohort was expanded to three additional patients. The MTD was defined as the highest dose level with DLTs seen in ≤1 of 6 patients.
After the MTD had been determined, 12 additional patients were treated with the MTD at the expansion cohort to assure tolerability. A decision rule based on Bayesian analysis dictated that ≤5 DLTs of 15 patients treated at a given dose level would define a dose acceptable for further study (13).
Patients treated at the expansion cohort also underwent tumor biopsies for pharmacodynamic evaluation. On day 1 of cycle 1, patients received irinotecan alone and underwent tumor biopsy 24 to 48 h later; on day 8 of cycle 1, patients received both irinotecan and 17AAG, with a second biopsy done 24 to 48 h after (see Supplementary Fig. S1 for study design schema). Because patients received only one combination treatment during cycle 1 at the MTD, DLT evaluation was extended to cycle 2 in this cohort to ensure adequate assessment of tolerability of the MTD.
Because of reported cardiopulmonary toxicities associated with 17AAG, the protocol was amended to include pulmonary function tests and an echocardiogram or multiple uptake gated acquisition scan at baseline. Laboratory evaluation for potassium, magnesium, and calcium (or ionized calcium) done within 24 h of each 17AAG treatment had to be within institutional normal limits. Any electrolyte disturbance necessitated correction to within the institutional reference range before the initiation of treatment. A post-17AAG infusion electrocardiogram was done during the 1st week of therapy in addition to that obtained at baseline.
Measurement of tumor indicator lesion(s) was done within 4 wk of beginning study treatment and every two cycles thereafter. Treatment responses were evaluated using response evaluation criteria in solid tumors (14).
Drug supply. Irinotecan (Camptosar; Pfizer), commercially available, was reconstituted in 5% dextrose solution. 17AAG (NSC 330507, National Cancer Institute) was prepared using the 2% DMSO/egg phospholipid diluent (NSC 704057, National Cancer Institute) as previously described (15).
Pharmacokinetics. During dose escalation, pharmacokinetic blood samplings were obtained during week 1 (cycle 1) when both drugs were given on the same day. Samplings were done at specified time points up to 24 h after the initiation of 17AAG. During week 1 (cycle 2), when irinotecan was given on day 1 and 17AAG on day 2, serial samples were collected at specified time points up to 24 h after the initiation of 17AAG.
Concentrations of 17AAG and its active metabolite 17-amino-17-demethoxy geldanamycin (17AG) in plasma were quantitated using a validated high-performance liquid chromatography assay (7). The time courses of 17AAG and 17AG in plasma were analyzed noncompartmentally. Pharmacokinetic variables were estimated using the LaGrange function (16), implemented by the LAGRAN computer program LAGRAN (16, 17).
The simultaneous assay of irinotecan, and its metabolites SN-38 and SN-38 glucuronideate (SN-38G), was used with slight modifications (18). Acetonitrile was used to remove proteins in the samples. A two-compartment model was fit to the data, and standard pharmacokinetic variables were calculated using WinNonlin software (Version. 5.2, Pharsight Corporation).
For pharmacokinetic interaction analysis, differences between the pharmacokinetic variables of 17AAG (or irinotecan) and its metabolites obtained with or without irinotecan (or 17AAG) coadministration were compared using a paired signed-rank test (paired Wilcoxon test). None of the expansion cohort patients were included in the pharmacokinetic data for irinotecan and its metabolites.
Pharmacodynamics. During cycle 1 in the expansion cohort, patients underwent tumor biopsies 24 to 48 h after irinotecan given alone on day 1, and after the combination of irinotecan and 17AAG on day 8. Tumor biopsy at baseline was optional. Up to three 18- to 20-gauge core needle biopsies were obtained under computer tomography guidance or using the Tru-Cut technique. Automated immunohistochemistry stainings were done on 5-μm formalin-fixed paraffin-embedded sections using established protocols on a Discovery XT processor (Ventana Medical Systems). Antibody binding was detected by the avidin-biotin peroxidase complex method (Vector Laboratories) using diaminobenzidine as a chromogen. Slides were counterstained with hematoxylin. The primary antibodies and their concentrations were Hsp70 (StressGene; 5 μg/mL), p-Chk1 (Ser 345; Cell Signaling; 2.5 μg/mL), p-H2AX (Ser 139; Upstate; 5 μg/mL), p-histone H3 (Ser 10; Upstate; 5 μg/mL), and cleaved caspase-3 (Cell Signaling; 0.1 μg/mL). P-Chk1 (Ser 345) was used as a surrogate for total Chk1 because commercially available antibodies for total Chk1 do not do well with immunohistochemistry. The expression of p-histone H3 was analyzed by immunofluorescence confocal microscopy using the antibody used for immunohistochemistry.
Sample sections were compared in a pairwise fashion by a pathologist (D.S.K.) who was blinded to the clinical data. For Hsp70, p-Chk1, and p-H2AX, immunoreactivity was evaluated semiquantitatively based on a staining score calculated by multiplying the percentage of positive tumor cells (0-100%) by the staining intensity (0, 1+, 2+, 3+, and 4+). For p-histone H3 and cleaved caspase-3, immunostaining was scored based on the number of positively stained tumor cells per high-powered field.
Analysis of p53 status. Tumor p53 status was determined on tissue sections from archived materials by immunohistochemistry using the monoclonal antibody (DO-7; DAKO; 1:500). Nuclear staining for p53 of >20% was considered indicative of mutant protein. The association between p53 status and treatment response was assessed using Fisher's exact test.
Results
Patient characteristics. Of 31 patients enrolled from May 2005 to March 2007, 27 received treatment. Four patients were ineligible because of prolonged QTc (n = 2) and elevated total bilirubin (n = 2; Table 1). Ten patients had previously received irinotecan.
Patient demographics
| Total no. of patients treated | 27 | |
| Median age, y (range) | 57 (32-73) | |
| Male to female ratio | 15:12 | |
| Median baseline KPS, % (range) | 80 (70-90) | |
| Median no. of prior chemotherapy treatments (range) | 3 (0-6) | |
| Primary disease sites | ||
| Colorectal | 6 | |
| Pancreatic/ampullary | 3 | |
| Breast | 3 | |
| Gastric | 2 | |
| Adrenocortical | 2 | |
| Sarcoma | 2 | |
| Gall bladder | 1 | |
| Anal | 1 | |
| Carcinoid | 1 | |
| Germ cell | 1 | |
| Head and neck | 1 | |
| High-grade neuroendocrine | 1 | |
| Liver | 1 | |
| Non–small cell lung | 1 | |
| Carcinoma of unknown primary | 1 | |
| Total no. of patients treated | 27 | |
| Median age, y (range) | 57 (32-73) | |
| Male to female ratio | 15:12 | |
| Median baseline KPS, % (range) | 80 (70-90) | |
| Median no. of prior chemotherapy treatments (range) | 3 (0-6) | |
| Primary disease sites | ||
| Colorectal | 6 | |
| Pancreatic/ampullary | 3 | |
| Breast | 3 | |
| Gastric | 2 | |
| Adrenocortical | 2 | |
| Sarcoma | 2 | |
| Gall bladder | 1 | |
| Anal | 1 | |
| Carcinoid | 1 | |
| Germ cell | 1 | |
| Head and neck | 1 | |
| High-grade neuroendocrine | 1 | |
| Liver | 1 | |
| Non–small cell lung | 1 | |
| Carcinoma of unknown primary | 1 | |
Abbreviation: KPS, Karnofsky performance status.
Sixty-seven cycles of treatment were given (median, 2; range, 1-12) over four dose levels. Twenty-six of 27 patients who commenced treatment were evaluable for toxicity. One patient in the MTD cohort withdrew consent after receiving one treatment and was replaced. Four patients were removed from the study because of DLTs during cycle 1, leaving 22 patients assessable for tumor response.
Dose escalation and toxicity. Common toxicities of ≥grade 2 that occurred in cycle 1 and all cycles and were at least possibly related to drug treatment are summarized in Tables 2 and 3, respectively. Patients enrolled in the first three cohorts completed cycle 1 without DLT. Four patients experienced DLTs in cohort 4 (irinotecan 100 mg/m2 and 17AAG 375 mg/m2). One patient developed grade 3 nausea, vomiting, and dehydration in part related to the odor from the DMSO used for solubilizing 17AAG. One patient experienced grade 3 diarrhea and dehydration, and another patient had grade 4 diarrhea. A fourth patient developed a symptomatic pulmonary embolism. This patient was managed with anticoagulation and continued study treatment at one lower dose level.
Incidence of grade 2 or higher adverse events (cycle 1 only)
| . | Cohort . | . | . | . | MTD . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | . | |||||||||||||||||
| CPT11, mg/m2 | 85 | 100 | 100 | 100 | 100 | |||||||||||||||||
| 17AAG, mg/m2 | 220 | 220 | 300 | 375 | 300 | |||||||||||||||||
| No. of patients | 3 | 3 | 3 | 5 | 13 | |||||||||||||||||
| Grades | ||||||||||||||||||||||
| Event | 2 | 3 | 2 | 3 | 2 | 3 | 2 | 3 | 4 | 2 | 3 | |||||||||||
| Hematologic | ||||||||||||||||||||||
| Leukopenia | — | — | 1 | — | 1 | — | — | 1 | — | 1 | — | |||||||||||
| Neutropenia | — | — | — | — | — | — | 1 | — | — | 1 | — | |||||||||||
| Anemia | 2 | — | 1 | — | 1 | — | 1 | — | — | 2 | — | |||||||||||
| Thrombocytopenia | — | — | — | — | — | — | — | — | — | — | — | |||||||||||
| Lymphopenia | — | — | — | — | — | — | — | 2 | — | — | 2 | |||||||||||
| Nonhematologic | ||||||||||||||||||||||
| Diarrhea | 1 | — | — | — | — | — | — | 1 | 1 | — | 1 | |||||||||||
| Fatigue | 1 | — | — | — | — | — | — | — | — | 2 | 1 | |||||||||||
| Nausea | — | — | — | — | — | — | 1 | 2 | — | 1 | — | |||||||||||
| Vomiting | — | — | — | — | — | — | 1 | 1 | — | 1 | — | |||||||||||
| Dehydration | — | — | — | — | — | — | 1 | 2 | — | — | — | |||||||||||
| Abdominal pain | — | — | — | — | — | — | — | — | — | — | 1 | |||||||||||
| Neuropathy | — | — | — | — | — | — | — | — | — | — | — | |||||||||||
| AST/ALT | — | — | — | — | — | — | — | — | — | — | — | |||||||||||
| Total bilirubin | — | — | — | — | — | — | 1 | — | — | — | — | |||||||||||
| Alkaline phosphatase | — | — | — | — | 2 | — | 1 | — | — | — | — | |||||||||||
| Thromboembolism | — | — | — | — | — | — | — | — | 1 | — | — | |||||||||||
| . | Cohort . | . | . | . | MTD . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | . | |||||||||||||||||
| CPT11, mg/m2 | 85 | 100 | 100 | 100 | 100 | |||||||||||||||||
| 17AAG, mg/m2 | 220 | 220 | 300 | 375 | 300 | |||||||||||||||||
| No. of patients | 3 | 3 | 3 | 5 | 13 | |||||||||||||||||
| Grades | ||||||||||||||||||||||
| Event | 2 | 3 | 2 | 3 | 2 | 3 | 2 | 3 | 4 | 2 | 3 | |||||||||||
| Hematologic | ||||||||||||||||||||||
| Leukopenia | — | — | 1 | — | 1 | — | — | 1 | — | 1 | — | |||||||||||
| Neutropenia | — | — | — | — | — | — | 1 | — | — | 1 | — | |||||||||||
| Anemia | 2 | — | 1 | — | 1 | — | 1 | — | — | 2 | — | |||||||||||
| Thrombocytopenia | — | — | — | — | — | — | — | — | — | — | — | |||||||||||
| Lymphopenia | — | — | — | — | — | — | — | 2 | — | — | 2 | |||||||||||
| Nonhematologic | ||||||||||||||||||||||
| Diarrhea | 1 | — | — | — | — | — | — | 1 | 1 | — | 1 | |||||||||||
| Fatigue | 1 | — | — | — | — | — | — | — | — | 2 | 1 | |||||||||||
| Nausea | — | — | — | — | — | — | 1 | 2 | — | 1 | — | |||||||||||
| Vomiting | — | — | — | — | — | — | 1 | 1 | — | 1 | — | |||||||||||
| Dehydration | — | — | — | — | — | — | 1 | 2 | — | — | — | |||||||||||
| Abdominal pain | — | — | — | — | — | — | — | — | — | — | 1 | |||||||||||
| Neuropathy | — | — | — | — | — | — | — | — | — | — | — | |||||||||||
| AST/ALT | — | — | — | — | — | — | — | — | — | — | — | |||||||||||
| Total bilirubin | — | — | — | — | — | — | 1 | — | — | — | — | |||||||||||
| Alkaline phosphatase | — | — | — | — | 2 | — | 1 | — | — | — | — | |||||||||||
| Thromboembolism | — | — | — | — | — | — | — | — | 1 | — | — | |||||||||||
NOTE: Numbers in bold indicate DLTs that occurred during cycle 1 in the dose escalation phase or during cycles 1 and 2 in the MTD expansion cohort. See text for DLT definitions.
Abbreviations: G, grade; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Incidence of grade 2 or higher adverse events (all cycles)
| . | Cohort . | . | . | . | MTD . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | . | |||||||||||||||
| Irinotecan, mg/m2 | 85 | 100 | 100 | 100 | 100 | |||||||||||||||
| 17AAG, mg/m2 | 220 | 220 | 300 | 375 | 300 | |||||||||||||||
| No. of patients | 3 | 3 | 3 | 5 | 13 | |||||||||||||||
| Grades | ||||||||||||||||||||
| Event | 2 | 4 | 2 | 2 | 3 | 2 | 3 | 4 | 2 | 3 | ||||||||||
| Hematologic | ||||||||||||||||||||
| Leukopenia | — | — | 1 | 1 | — | — | 1 | — | 5 | — | ||||||||||
| Neutropenia | — | — | — | 1 | — | 1 | — | — | 1 | 2 | ||||||||||
| Anemia | 3 | — | 1 | 1 | 1 | 1 | — | — | 4 | — | ||||||||||
| Thrombocytopenia | — | — | — | — | — | — | — | — | — | — | ||||||||||
| Lymphopenia | — | — | — | — | — | — | 2 | — | — | 3 | ||||||||||
| Nonhematologic | ||||||||||||||||||||
| Diarrhea | 1 | — | — | — | — | — | 1 | 1 | 2 | 2 | ||||||||||
| Fatigue | 2 | — | — | — | 1 | — | — | — | 2 | 2 | ||||||||||
| Nausea | — | — | — | — | — | 1 | 2 | — | 3 | — | ||||||||||
| Vomiting | — | — | — | — | — | 1 | 1 | — | 2 | — | ||||||||||
| Dehydration | — | — | — | — | — | 1 | 2 | — | 1 | — | ||||||||||
| Abdominal pain | — | — | — | — | — | — | — | — | 2 | 1 | ||||||||||
| Neuropathy | — | — | — | — | — | — | — | — | 1 | — | ||||||||||
| AST/ALT | — | — | — | 1 | — | — | — | — | — | — | ||||||||||
| Total bilirubin | — | — | — | 1 | — | 1 | — | — | — | — | ||||||||||
| Alkaline phosphatase | — | — | — | 1 | 1 | 1 | — | — | — | — | ||||||||||
| Thromboembolism | — | 1 | — | — | — | — | — | 1 | — | — | ||||||||||
| . | Cohort . | . | . | . | MTD . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | . | |||||||||||||||
| Irinotecan, mg/m2 | 85 | 100 | 100 | 100 | 100 | |||||||||||||||
| 17AAG, mg/m2 | 220 | 220 | 300 | 375 | 300 | |||||||||||||||
| No. of patients | 3 | 3 | 3 | 5 | 13 | |||||||||||||||
| Grades | ||||||||||||||||||||
| Event | 2 | 4 | 2 | 2 | 3 | 2 | 3 | 4 | 2 | 3 | ||||||||||
| Hematologic | ||||||||||||||||||||
| Leukopenia | — | — | 1 | 1 | — | — | 1 | — | 5 | — | ||||||||||
| Neutropenia | — | — | — | 1 | — | 1 | — | — | 1 | 2 | ||||||||||
| Anemia | 3 | — | 1 | 1 | 1 | 1 | — | — | 4 | — | ||||||||||
| Thrombocytopenia | — | — | — | — | — | — | — | — | — | — | ||||||||||
| Lymphopenia | — | — | — | — | — | — | 2 | — | — | 3 | ||||||||||
| Nonhematologic | ||||||||||||||||||||
| Diarrhea | 1 | — | — | — | — | — | 1 | 1 | 2 | 2 | ||||||||||
| Fatigue | 2 | — | — | — | 1 | — | — | — | 2 | 2 | ||||||||||
| Nausea | — | — | — | — | — | 1 | 2 | — | 3 | — | ||||||||||
| Vomiting | — | — | — | — | — | 1 | 1 | — | 2 | — | ||||||||||
| Dehydration | — | — | — | — | — | 1 | 2 | — | 1 | — | ||||||||||
| Abdominal pain | — | — | — | — | — | — | — | — | 2 | 1 | ||||||||||
| Neuropathy | — | — | — | — | — | — | — | — | 1 | — | ||||||||||
| AST/ALT | — | — | — | 1 | — | — | — | — | — | — | ||||||||||
| Total bilirubin | — | — | — | 1 | — | 1 | — | — | — | — | ||||||||||
| Alkaline phosphatase | — | — | — | 1 | 1 | 1 | — | — | — | — | ||||||||||
| Thromboembolism | — | 1 | — | — | — | — | — | 1 | — | — | ||||||||||
Cohort 3 (irinotecan 100 mg/m2 and 17AAG 300 mg/m2) was therefore designated as the MTD. We expanded this cohort to enroll 12 additional patients. One patient withdrew consent after developing grade 2 gastrointestinal toxicities before undergoing the second tumor biopsy; this patient was replaced. Four patients experienced DLTs, including one grade 3 diarrhea, one grade 3 abdominal pain, one grade 3 fatigue, and one with dose delay ≥7 days because of grade 1 thrombocytopenia. Thus, the incidence of protocol-defined DLT was 4 of 15 patients (27%) treated at the MTD. Using a Bayesian approach, the probability that DLT rate exceeds 33% is calculated to be 0.34, within acceptable limits of toxicity tolerance in phase I trials. There is a 95% probability that the true DLT rate is between 0.11 and 0.52 (13).
Among other significant toxicities, grade 2 hepatotoxicity was noted in one patient during cycle 2. One patient developed dyspnea after four cycles of treatment and was found to have a pulmonary embolism. One patient experienced grade 3 fatigue during cycle 3, and one patient had grade 2 (but progressive) neuropathy that required discontinuation of therapy after two cycles. Post-17AAG infusion electrocardiograms done during the 1st week of cycle 1 showed no cardiac dysrhythmias.
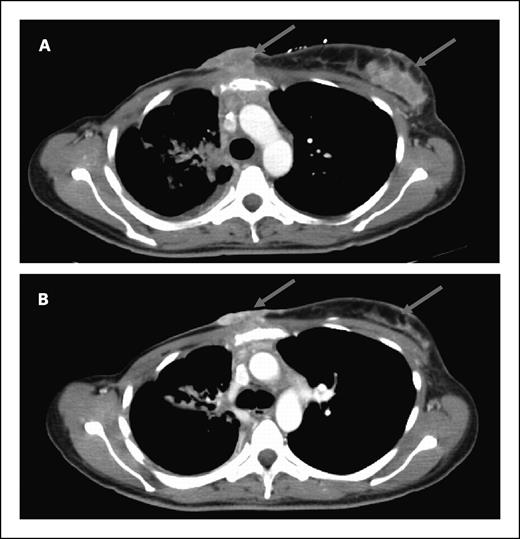
Antitumor activity. Of the 22 accessible patients, none had a complete or partial response by response evaluation criteria in solid tumors. Eleven patients had stable disease as the best response. The median duration of stable disease was 3 months (range, 1.5-8 months). One patient with Her2-negative breast cancer had a 29% reduction in target lesions after two cycles (Fig. 1). Minor responses in target lesions were seen in four other patients: non–small cell lung (−13%), head and neck squamous cell (−10%), colorectal (−9%), and pancreatic adenocarcinoma (−8%). One patient with ampullary adenocarcinoma had improvement of carcinomatosis and a decrease in CA19-9 from 1,346 to 174 units/mL. All patients who showed tumor measurement reductions were irinotecan-naïve. One patient with irinotecan-refractory colorectal cancer was treated on the current study for five months. Tumor p53 status was available for 16 patients. Two of six patients with wild-type p53 had stable disease compared with 5 of 10 patients with mutant p53 (P = 0.63; Fisher's exact test).
Computed tomography of a patient with Her2-negative p53-mutant metastatic breast cancer before (A) and after two cycles (B) of 17AAG and irinotecan given at the MTD, showing regression of chest wall metastases (arrows).
Computed tomography of a patient with Her2-negative p53-mutant metastatic breast cancer before (A) and after two cycles (B) of 17AAG and irinotecan given at the MTD, showing regression of chest wall metastases (arrows).
Pharmacokinetic studies. During dose escalation, pharmacokinetic samplings were done both in cycle 1 when irinotecan and 17AAG were given on the same day, and in cycle 2 when the two agents were given sequentially on days 1 and 2. Pairwise comparison of the pharmacokinetic variables of 17AAG and 17AG obtained between the two cycles for each patient showed no significant differences, suggesting that the pharmacokinetic of 17AAG was not affected by irinotecan coadministration (Supplementary Table S1). Likewise, the pharmacokinetic variables of irinotecan, SN-38, and SN-38G obtained during cycle 1 were not significantly different from those obtained in cycle 2, indicating no effect of 17AAG treatment on the pharmacokinetic of irinotecan (Supplementary Table S2). The mean plasma concentration versus time curves of 17AAG and 17AG in patients treated at the MTD are shown in Supplementary Fig. S2. The mean area under the curve, half-life, and clearance for 17AAG are 35.9 ± 15.9 μmol/L hours, 2.0 ± 1.2 hours, and 17.1 ± 10.6 L/h/m2, respectively. The mean area under the curve and half-life of 17AG are 36.7 ± 21.2 μmol/L hours and 2.3 ± 1.5 hours, respectively. These values agree with those reported in two phase I studies of single-agent 17AAG given at a similar dose in one of their cohorts (6, 19).
Pharmacodynamic analyses. During the first cycle of the MTD expansion cohort, 12 additional patients underwent tumor biopsies after treatment with irinotecan only in week 1 and with irinotecan and 17AAG in week 2. All but one paired set of biopsies were obtained within 24 hours after drug treatment. There were no biopsy-related complications. Eight of 12 paired samples contained tumor cells deemed adequate for pharmacodynamic evaluation. Markers were examined for these cellular effects: (a) Hsp90 inhibition as measured by induction of the cochaperone Hsp70; (b) loss of p-Chk1 as a surrogate for depletion of the Hsp90 client protein, Chk1; (c) abrogation of the G2-M checkpoint as measured by staining of the mitosis-specific marker p-histone H3; (d) p-H2AX for DNA damage response; and (e) cleaved caspase-3 for apoptosis. Hsp70 was up-regulated following treatment with irinotecan and 17AAG in six of eight patients (Fig. 2). Of the six patients whose tumors showed an induction of the cochaperone, four also showed a decrease in p-Chk1 (Fig. 2). The expression of p-H2AX was elevated following combination treatment in three patients, two of whom also had Hsp70 induction and p-Chk1 down-regulation (Fig. 2). An increase in p-histone H3 was shown in two patients whereas an increase in cleaved caspase-3 was found in three patients (Fig. 2). Figure 3A illustrates a case of p53-mutant (positive p53 staining) gastric cancer metastasized to the liver in which Hsp90 inhibition was associated with a depletion of its client Chk1, leading to an abrogation of the G2-M checkpoint, as well as an increase in DNA damage and apoptosis. Examination of the nuclear morphology of tumor cells under fluorescence microscopy showed mitotic cells with fragmented chromatin characteristic of cells undergoing mitotic catastrophe in the postcombination sample (Fig. 3B; ref. 20).
Semiquantitative analysis of changes in pharmacodynamic markers in tumor biopsy samples obtained from patients treated at the MTD after irinotecan (CPT11) alone and after irinotecan and 17AAG (CPT11+17AAG). Different marker studies done for the same patient are represented by the same symbol. HPF, high power field.
Semiquantitative analysis of changes in pharmacodynamic markers in tumor biopsy samples obtained from patients treated at the MTD after irinotecan (CPT11) alone and after irinotecan and 17AAG (CPT11+17AAG). Different marker studies done for the same patient are represented by the same symbol. HPF, high power field.
A, immunohistochemistry analysis of paired biopsies in a patient with p53-mutant gastric adenocarcinoma, showing in the postcombination (CPT11+17AAG) samples an induction of Hsp70, a suppression of p-Chk1 nuclear staining, an increase in p-H2AX nuclear staining, an increase in p-histone H3 indicative of G2-M checkpoint abrogation, as well as an increase in cleaved caspase-3. Scale bar, 100 μm. B, confocal immunofluorescence microscopy of tumor samples stained for p-histone H3 (pH3; green) showing tumor cells with fragmented chromatin characteristic of those undergoing mitotic catastrophe after irinotecan and 17AAG treatment (arrows). Chromatin was counterstained using 4′-6-diamidino-2-phenylindole (DAPI; blue). Scale bar, 20 μm.
A, immunohistochemistry analysis of paired biopsies in a patient with p53-mutant gastric adenocarcinoma, showing in the postcombination (CPT11+17AAG) samples an induction of Hsp70, a suppression of p-Chk1 nuclear staining, an increase in p-H2AX nuclear staining, an increase in p-histone H3 indicative of G2-M checkpoint abrogation, as well as an increase in cleaved caspase-3. Scale bar, 100 μm. B, confocal immunofluorescence microscopy of tumor samples stained for p-histone H3 (pH3; green) showing tumor cells with fragmented chromatin characteristic of those undergoing mitotic catastrophe after irinotecan and 17AAG treatment (arrows). Chromatin was counterstained using 4′-6-diamidino-2-phenylindole (DAPI; blue). Scale bar, 20 μm.
Of the limited number of patients with paired biopsies who were evaluable for tumor response (n = 7), we found no correlation between changes of biomarkers and treatment response (see Discussion).
Discussion
Both Hsp90 and Chk1 have emerged as novel anticancer targets. Of considerable interest to both therapeutic areas is the identification of Chk1 as a Hsp90 client protein (11). We and others have shown that treatment with 17AAG results in depletion of cellular Chk1 and abrogation of the S and/or G2-M checkpoints induced by chemotherapy, prompting us to embark on this phase I study of 17AAG and irinotecan7
Tse AN, Sheikh T, Ho A., Chou TC, et al. Hsp90 inhibition abrogates the G2/M checkpoint in p53-null tumor cells by depleting Chk1 and Wee1. Mol. Pharmacol. Accepted for publication.
In three single-agent phase I studies, the MTD of 17AAG given once weekly or thrice weekly in a 4-week cycle (two studies) was determined to be 450, 308, and 295 mg/m2, respectively (5–7). In the current combination study, the recommended phase II dose is 300 mg/m2 17AAG and 100 mg/m2 irinotecan. Thus, 17AAG can be delivered at essentially its full singe-agent dose. As predicted from the toxicity profile of each individual agent, gastrointestinal toxicities are dose-limiting for the combination. It is possible that some of these adverse effects are from the DMSO required for formulating 17AAG.
No complete or partial responses were noted in this study. However, one patient with breast cancer showed a 29% reduction in index lesions, and five other patients showed tumor shrinkage. Although it may be difficult to separate the activity of the combination from that of irinotecan alone, as all patients who showed tumor shrinkage were irinotecan-naïve, we were encouraged to see signs of antitumor activity in this heavily pretreated group. Preclinical studies have suggested that tumors with intrinsic checkpoint defects, by virtue of p53 loss, may be more susceptible to undergoing G2-M checkpoint abrogation and apoptosis induced by treatment with 17AAG and irinotecan. In our trial, two of six patients with wild-type p53 had stable disease compared with 5 of 10 patients with mutant p53. The relationship between tumor p53 status and response to this drug combination therefore deserves further exploration. Given the encouraging response data seen in a recently completed phase II study of 17AAG and trastuzumab in trastuzumab-refractory Her2-positive breast cancer (21) and the 29% tumor shrinkage observed in a patient with Her2-negative disease in the current study, breast cancer would potentially be a tumor type in which the combination of irinotecan and 17AAG could be further developed.
Validation of the pharmacodynamic effects of Hsp90 inhibition presents a unique challenge in this combination study, as it requires showing the successful suppression of the Hsp90 client Chk1 and abrogation of the irinotecan-induced G2-M checkpoint as a result of Chk1 inhibition. Pharmacodynamic evaluations at the MTD were designed to discern the biological effects of combined 17AAG and irinotecan (week 2) from that of irinotecan alone (week 1). One caveat is that it may not be possible to determine whether the biomarker changes seen in the second biopsy are from 17AAG or a delayed effect of irinotecan given in the previous week. However, considering the short-lived nature of the cellular processes relevant to G2-M checkpoint abrogation (i.e., mitosis and apoptosis) and the advantage of using the patient as his or her own control, this concern is probably minor. Ideally, a third biopsy obtained at baseline (optional for the study) would allow assessment of the pharmacodynamic effects induced by irinotecan alone. However, we were unable to obtain any baseline biopsies because of logistic reasons.
In preclinical studies, a 24-hour exposure of tumor cells in culture to 200 to 500 nmol/L 17AAG results in Chk1 depletion and abrogation of the G2-M checkpoint induced by SN-38.7 At the MTD, the mean combined plasma concentration of 17AAG and its active metabolite 17AG measured at 24 hours was ∼300 nmol/L, suggesting that biologically relevant drug concentrations can be achieved in some patients. This was supported by pharmacodynamic validation studies. Of the biomarkers examined, Hsp70 induction was mostly consistently seen after 17AAG treatment (6 of 8 patients). Chk1, the Hsp90 client of interest, was down-regulated in four samples. Evidence for abrogation of the G2-M checkpoint, as measured by induction of the M-phase specific marker p-histone H3, was shown in two patients, both of whom were mutant for p53. To our knowledge, these results show for the first time the biological effects of a Hsp90 inhibitor causing down-regulation of the client protein, Chk1, and abrogation of the G2-M checkpoint in human tumors.
Of note, the patient with the pharmacodynamic responses shown in Fig. 3 was taken off the study for “early disease progression” after experiencing increased fatigue and abdominal pain during cycle 1. An imaging study done after only one combination treatment of irinotecan and 17AAG revealed tumor progression in the liver. However, retrospective examination of the computer tomography done for tumor biopsy after irinotecan alone showed already substantial tumor progression when compared with baseline. It is possible that the pharmacodynamic effects observed in this patient preceded subsequent radiologic response if this patient was allowed to continue on study.
In summary, the combination of irinotecan and 17AAG can be given with acceptable toxicity. At this juncture, further clinical development of this combination is unclear as non-DMSO formulation of 17AAG and newer generations of Hsp90 as well as more selective Chk1 inhibitors are now available (22, 23). Nonetheless, our pharmacodynamic studies show for the first time that Chk1-mediated signaling can be disrupted by a Hsp90 inhibitor in human tumors, providing the proof-of-mechanism of this therapeutic approach. We envisioned that this strategy of combining cytotoxic agents with checkpoint inhibitor can be further optimized with improved formulation of 17AAG and/or selective Chk1 inhibitors that are currently undergoing clinical testing.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Grant support: Grants K08CA116612 (A.N. Tse), U01CA069856, UO1CA099168 (M.J. Egorin), and 2P30 CA47904 (M.J. Egorin) from the National Cancer Institute, and a Career Development Award from the American Society of Clinical Oncology (A.N. Tse).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Acknowledgments
We thank the members of the Molecular Cytology Core Facility at Memorial Sloan-Kettering Cancer Center for their technical assistance in immunohistochemistry and immunofluorescence analysis, and Carol Pearce for her editorial assistance.