Abstract
Objective: There is general agreement that the testing protocol for measuring cigarette smoke constituents—the International Organization for Standardization regimen—is an inappropriate mechanism for evaluating human exposure. Alternative smoking regimens have been introduced in Canada and Massachusetts; however, these regimens have not been evaluated against human smoking behavior and biomeasures of exposure. The objective of this study was to compare measures of smoke volume and nicotine uptake among human smokers against the puffing variables and nicotine yields generated by five different machine smoking regimens: (a) International Organization for Standardization, (b) Massachusetts, (c) Canadian, (d) a Compensatory regimen, and (e) a Human Mimic regimen.
Methods: Measures of smoke volume and puffing behavior were recorded for 51 smokers who used a portable smoking topography device for three 1-week trials. Measures of salivary cotinine were taken at the completion of each week. The cigarette brands smoked by participants were then machine-smoked under five testing regimens, including a human mimic condition where brands were machine smoked using the puffing behavior recorded from human smokers. The total volume of smoke collected from each cigarette and the nicotine, tar, and carbon monoxide yields were recorded.
Results: None of the four machine smoking regimens adequately reflected Human Mimic Yields of tar, nicotine, and carbon monoxide. In addition, none of the four smoking regimens generated nicotine yields that were associated with actual nicotine uptake in humans.
Conclusions: None of the existing smoking regimens adequately represents human smoking behavior nor do they generate yields associated with human measures of nicotine uptake. (Cancer Epidemiol Biomarkers Prev 2006;15(8):1495–501)
Introduction
The toxicity of cigarette smoke is determined by a complex set of product characteristics, including the tobacco blend and additives, as well as design features such as filter ventilation and paper porosity (1). To date, the primary means of testing cigarette toxicity has been to machine-smoke cigarettes according to a standard puffing regimen and to measure the constituents in the mainstream smoke. The protocol for machine smoking was adopted by the Federal Trade Commission (FTC) in 1967 and soon after by the International Organization for Standardization (ISO; ref. 2). The FTC/ISO testing regimens are mandatory in many countries and form the basis for the tar and nicotine yields that are communicated to consumers via tobacco advertising and/or cigarette packs. ISO yields also serve as a regulatory limit in a number of jurisdictions, including the European Union, where brands that generate yields >10 mg tar, 1 mg nicotine, or 10 mg carbon monoxide (CO) are prohibited.
However, there are serious limitations to the FTC/ISO smoking regimens. The FTC/ISO puffing variables have been shown to systematically underestimate the size, frequency, and velocity of puffs for most human smokers, including those who smoke “regular” yield, low-ventilation brands (3-6). In fact, the originators of the FTC/ISO method from the American Tobacco Company noted as early as 1936 that their method did not represent human smoking habits (7). The FTC/ISO method also did not account for compensatory smoking behavior, whereby human smokers regulate their intake by changing their puffing behavior to adjust for differences in nicotine delivery: Whereas human smokers increase the intensity of their puffing when smoking “low-yield” cigarettes, the FTC/ISO regimen smokes all cigarettes using the same puffing conditions (8-12). In addition, cigarette manufacturers have designed cigarettes to perform one way under machine testing, but to deliver much higher levels of nicotine and other smoke constituents in human hands (12, 13). Filter ventilation—tiny perforations in cigarette filters that allow air to enter and dilute the cigarette smoke collected under machine smoking conditions—is the most prominent design element responsible for this discrepancy, but by no means the only one (12). As a consequence, the FTC/ISO machine yields bear little association with biological measures of uptake among human smokers (14-16).
There is an urgent need to revise the existing testing protocols. The Framework Convention on Tobacco Control—the first international public health treaty—includes provisions for testing and regulating cigarette emissions (17). Although there is strong consensus within the public health community that the ISO regimen is inadequate for the purposes of product regulation, there is little consensus regarding an alternative that could be recommended to the 126 countries that have ratified the Framework Convention on Tobacco Control to date (18-21). An ISO Working Group (ISO/TC126/WG9) is currently reviewing options for a machine smoking regimen that is more representative of human smoking behavior. Meanwhile, WHO Study Group on Tobacco Product Regulation (TobReg) has endorsed a smoking regimen that represents “intensive” smoking patterns (20).
Two jurisdictions have introduced alternative machine smoking regimens to supplement the FTC/ISO cigarette yields. The Commonwealth of Massachusetts in the United States currently tests cigarettes with a 45 mL puff drawn twice per minute, with 50% of the filter vent holes blocked. In contrast, Canadian testing standards require both the ISO regimen, as well as an intensive smoking regimen, where 55 mL puffs are drawn twice per minute with 100% of vent holes blocked (see Table 1; refs. 22, 23).
A description of international cigarette testing standards
| . | Vent hole blockage (%) . | Interpuff interval (s) . | Mean flow rate (mL/s) . | Puff volume (mL) . |
|---|---|---|---|---|
| ISO | 0 | 60 | 17.5 | 35 |
| Massachusetts | 50 | 30 | 22.5 | 45 |
| Canadian | 100 | 30 | 27.5 | 55 |
| Compensatory model* | 50 | 30 | 20.0 | 40 |
| . | Vent hole blockage (%) . | Interpuff interval (s) . | Mean flow rate (mL/s) . | Puff volume (mL) . |
|---|---|---|---|---|
| ISO | 0 | 60 | 17.5 | 35 |
| Massachusetts | 50 | 30 | 22.5 | 45 |
| Canadian | 100 | 30 | 27.5 | 55 |
| Compensatory model* | 50 | 30 | 20.0 | 40 |
NOTE: All options include ISO puff durations (2 seconds) and butt length (filter length +8 mm, or filter overwrap +3 mm, whichever is greater). Note that the FTC and ISO regimens are the same except for butt length (FTC = 23 mm butt length, or filter + 3 mm).
The values are for cigarettes with 1.0 mg (ISO) nicotine only. For all other brands, the puff volume and frequency of puffs increases as the ISO nicotine yield decreases.
As an alternative, Kozlowski and O'Connor (19) have proposed a “compensatory” machine smoking regimen. Rather than smoking all brands using the same puffing regimen, the Compensatory regimen attempts to mimic the systematic differences in human smoking across different products, whereby lower nicotine yield brands are smoked more intensely. Kozlowski and O'Connor advocate varying the puff volume and puff frequency according to the ISO nicotine yield. For brands with <10 mg tar, a 40 mL puff is taken every 60 seconds. With every decrease of 0.1 mg nicotine, the puff volume rises by 4 mL and the puff frequency increases by 4 seconds. For example, a cigarette with 0.5 mg nicotine under the ISO method would be smoked at 60 mL puffs every 40 seconds, whereas a 0.1 mg cigarette would be smoked at 76 mL puffs every 24 seconds. Table 1 compares the ISO, Massachusetts, Canadian, and Compensatory machine smoking regimens.
To our knowledge, the Massachusetts, Canadian, and Compensatory regimens have not been evaluated with respect to human smoking behavior. Therefore, it is unclear to what extent the yields from these alternative regimens are any better at representing human puffing variables or predicting measures of human exposure to smoke constituents. The purposes of this study were as follows: (a) to characterize puffing behavior and the volume of smoke exposure among human smokers for a range of Canadian cigarette brands; (b) to test each brand under the ISO, Massachusetts, Canadian, Compensatory, and Human Mimic machine smoking regimens and to measure the total smoke volume and nicotine yields generated by each regimen; and (c) to compare the nicotine yields from each regimen with measures of nicotine uptake among human smokers.
Materials and Methods
Fifty-nine participants (51% male, mean age 37.1 years, mean cigarettes per day 19.3) completed a field study of smoking behavior using portable CreSSmicro devices (Plowshare Technologies, Inc. Baltimore, Maryland). Eligibility to participate in the study was limited to individuals who reported smoking a minimum of five cigarettes per day, had no intention to quit smoking in the next 3 months (the duration of the study period), and who smoked 1 of 17 brands with ISO tar yields between 9 and 15 mg.
The full-field study protocol is described elsewhere (24). Briefly, the field study consisted of three 1-week trials over a 2-month period. For each trial, participants smoked at least five cigarettes a day through the portable smoking topography device for 5 consecutive days. CReSSmicro is a battery-operated portable device that measures a full complement of smoking topography variables (puff volume, puff count, puff duration, peak flow, interpuff interval, time, and date).
Participants smoked their usual brand of cigarettes during trial 1 and, again, 6 weeks later, during trial 2. Trial 3 occurred during the week immediately following trial 2. For trial 3, half of the participants were randomly selected to smoke a “lower-yield” cigarette brand (Matinee Extra Mild, 4 mg tar/0.4 mg nicotine ISO yield), whereas half continued to smoke their usual brand. The lower-yield brand was matched for length and diameter with usual brand cigarettes. All participants were provided with cigarettes for trial 3—either their regular brand or the lower-yield brand—free of charge. Participants were offered $60 CDN for completing each of three 1-week trials, for a maximum of $180 CDN. The study protocol was cleared for ethics by the Research Ethics Board of the University of Waterloo and the Institutional Review Board of the Roswell Park Cancer Institute.
Salivary Cotinine
Cotinine is the major metabolite of nicotine with an average half-life of ∼20 hours and is a reliable indicator of nicotine uptake (25-27). Immediately following each smoking trial, participants were asked to provide a saliva sample, which was then frozen for storage. The saliva samples were analyzed for cotinine by Labstat International, Inc. (Kitchener, Ontario), using a rapid gas-liquid chromatographic method (28).
Cigarette Testing
Samples of each of the cigarette brands smoked by 51 of the 59 subjects in this study were purchased and sent to Arista Laboratories (Richmond, VA) to measure the tar, nicotine, and CO yields. (Note that brands smoked by eight participants in the original field study were excluded due to a data processing error.) Four replicates (five cigarettes per replicate) of each brand were smoked through a Filtrona smoking machine under the ISO, Massachusetts, Canadian, Compensatory, and Human Mimic smoking regimens. Puff count, nicotine, tar, and CO yields were determined using the same standard methodology for all five protocols. Particulate matter from the mainstream smoke was collected on a 44-mm Cambridge filter pad from each smoking port, which was then removed and weighed to measure total particulate matter. Extract from the filter pad was injected into a gas chromatograph to determine the moisture content and nicotine yield (29). Gases that passed through the filter pad were collected and then tested to determine carbon monoxide yield (30). Tar level was determined by subtracting the water and nicotine levels from the total particulate matter (31). Puff count was recorded to the first decimal point.
Human Mimic Smoking Regimen
The Filtrona machine was programmed to smoke cigarettes using the puffing behavior recorded from respondents in the field study. In cases where a particular brand was smoked by more than one subject, the mean puffing level was used. In addition, all brands were machine smoked with 50% of the filter covered, based on previous population-based estimates of vent blocking (32). This human mimic smoking regimen generates cigarette yields under realistic human smoking conditions that can serve as a benchmark for evaluating the yields from standard smoking regimens. Note that Human Mimic Yields have been used extensively by tobacco manufacturers as a product testing benchmark (13).
Analysis
The current analysis was conducted with 51 participants who participated in the field study and for which complete data on all key measures was available. All analyses were conducted using SPSS (version 12.0, Chicago, IL). A measure of “smoke volume” was computed by multiplying the total number of puffs per cigarette by the mean puff volume. Thus, smoke volume was a measure of the total smoke drawn from a cigarette, either by human smokers or by the smoking machine. A linear regression model was run to examine predictors of salivary cotinine. In step 1, gender, cigarettes per day, smoke volume, and the time of day the salivary cotinine sample was collected were entered into the model. A two-way interaction between cigarettes per day and smoke volume that was significantly associated with cotinine in previous analyses of the same data (24) was also included in the model. In step 2 of the model, nicotine yields were entered as a predictor variable. Step 2 was conducted separately for each of the machine smoking regimens examined in the current study. Results are shown for data from trial 1.
Results
The 51 study participants smoked a total of 5,409 cigarettes through the CReSSmicro device over the course of the study, >50% of all cigarettes smoked. There was no difference between the control and brand-switching groups in the percentage of cigarettes smoked through the CReSSmicro device (P > 0.10).
Differences in Smoke Volume between Humans and Machine Smoking Regimens
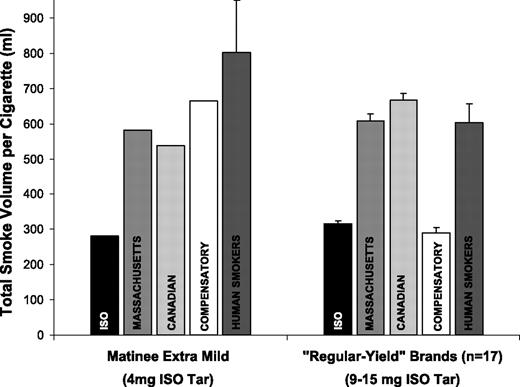
The puffing variables recorded from study participants have been reported elsewhere (24). The 51 participants in the current analysis took an average of 53.3 mL puffs (SD 11.9), 11.5 puffs per cigarette (SD 3.6), with a mean puff frequency of 33.2 seconds (SD 15.8), while smoking their usual cigarette brand at trials 1 and 2. Each puff was drawn for an average of 1.4 seconds (SD 0.3), with an average flow rate of 38.6 mL/s (SD 5.9). Overall, participants drew a total volume of 602.6 mL of smoke per cigarette (SD 195.8) when smoking their usual brand. The 21 participants in the current analysis who were “switched” to Matinee Extra Mild at trial 3 drew a total volume of 802.4 mL smoke from each cigarette (SD 346.2), with an average of 60.7 mL puffs (SD 15.6), 13.4 puffs per cigarette (SD 5.3), with a puff frequency of 30.5 seconds (SD 15.4), an average puff duration of 1.6 seconds (SD 0.4), and puffs at an average flow rate of 40.8 mL/s (SD 8.4). Figure 1 shows the total smoke volume drawn by participants compared with the average total smoke volume drawn from the same brands by the Canadian, Massachusetts, Compensatory, and ISO machine testing regimens.
Total smoke volume per cigarette: Human smokers versus four machine smoking regimes.
Total smoke volume per cigarette: Human smokers versus four machine smoking regimes.
Among “regular-yield” brands, the volume of smoke generated by the Canadian regimen (mean 667.1 mL, SD 69.9) was significantly greater than human smokers (P = 0.024), whereas the total smoke volume generated by the Massachusetts regimen (mean 608.5 mL, SD 68.8) was not significantly different. The total smoke volume for the ISO (mean 315.0 mL, SD 36.0) and Compensatory regimens (mean 289.1 mL, SD 56.9) were significantly lower than human smokers (P < 0.001 in both cases). For the Matinee Extra Mild brand smoked at trial 3, all four smoking regimens underestimated the total smoke volume compared with the human smokers, as indicated in Fig. 1. It should also be noted that the average flow rate of human smokers for both regular (38.6 mL/s) and low-yield brands (40.8 mL/s) was considerably greater than the flow rate for the ISO (17.5 mL/s), Massachusetts (22.5 mL/s), Canadian (27.5 mL/s), and Compensatory regimens (20.0 mL/s).
Human Mimic Regimen
The Human Mimic machine smoking regimen was used to generate cigarette yields under similar puffing conditions as those observed among human smokers in the field study. As a result, the smoking machine was programmed to smoke each brand using the mean puff volume and puff frequency recorded from human smokers for each brand. There was a modest discrepancy between the total smoke volume recorded from human smokers and the total smoke volume produced by the Human Mimic regimen: Whereas human smokers drew an average of 602.6 mL smoke from each cigarette, the Human Mimic regimen drew an average of 671.4 mL (SD 147.7) smoke for regular-yield brands. This represents an overestimate of ∼11.4% percent across brands. For the low-yield Matinee Extra Mild brand at trial 3, human smokers drew an average of 802.4 mL smoke compared with 725.0 mL under the Human Mimic regimen or 9.6% less smoke than humans. This discrepancy was the result of different puff counts taken by participants and the smoking machine while conducting the Human Mimic testing regimen. (The puff number of the Human Mimic regimen was not fixed. Rather, the smoking machine was programmed to use puff frequencies recorded from human smokers, and to continue drawing puffs until the cigarette butt reached 23 mm in length—the ISO standard.).
Human Mimic versus Standard Machine Yields
Table 2 compares the cigarette yields generated by the different smoking regimens. As Table 2 indicates, the Human Mimic yields were approximately double those generated by the ISO and Compensatory regimens (P < 0.001 in all cases) for the 17 regular-yield brands. The Canadian regimen generated greater tar (P = 0.03), nicotine (P = 0.01), and CO yields (P = 0.07) than the Mimic regimen, whereas none of yields from the Massachusetts regimen were significantly different from the Mimic yields. There were no significant differences between the Compensatory and ISO yields for regular-yield brands.
Differences in cigarette yields according to testing regimen
| . | ISO . | Massachusetts . | Canadian . | Compensatory . | Human Mimic . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Regular-yield brands (9-15 mg ISO tar) | ||||||||||
| Nicotine (mg) | 1.1 (0.1) | 2.3 (0.2) | 2.4 (0.3) | 1.0 (0.1) | 2.0 (0.6) | |||||
| Tar (mg) | 12.2 (1.8) | 26.0 (3.2) | 30.6 (3.1) | 11.6 (1.9) | 24.7 (9.5) | |||||
| CO (mg) | 12.2 (2.0) | 23.8 (2.9) | 28.1 (3.8) | 11.4 (1.7) | 24.6 (7.1) | |||||
| Matinee extra mild (4 mg ISO tar)* | ||||||||||
| Nicotine (mg) | 0.4 | 1.2 | 1.6 | 1.4 | 1.5 | |||||
| Tar (mg) | 4.9 | 12.5 | 22.9 | 13.7 | 16.6 | |||||
| CO (mg) | 4.4 | 13.4 | 22.9 | 13.8 | 19.2 | |||||
| . | ISO . | Massachusetts . | Canadian . | Compensatory . | Human Mimic . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Regular-yield brands (9-15 mg ISO tar) | ||||||||||
| Nicotine (mg) | 1.1 (0.1) | 2.3 (0.2) | 2.4 (0.3) | 1.0 (0.1) | 2.0 (0.6) | |||||
| Tar (mg) | 12.2 (1.8) | 26.0 (3.2) | 30.6 (3.1) | 11.6 (1.9) | 24.7 (9.5) | |||||
| CO (mg) | 12.2 (2.0) | 23.8 (2.9) | 28.1 (3.8) | 11.4 (1.7) | 24.6 (7.1) | |||||
| Matinee extra mild (4 mg ISO tar)* | ||||||||||
| Nicotine (mg) | 0.4 | 1.2 | 1.6 | 1.4 | 1.5 | |||||
| Tar (mg) | 4.9 | 12.5 | 22.9 | 13.7 | 16.6 | |||||
| CO (mg) | 4.4 | 13.4 | 22.9 | 13.8 | 19.2 | |||||
No SDs are listed given that only one low-yield brand was tested in the current study.
A similar pattern of results was observed for the Matinee Extra Mild brand, smoked by half of the participants at trial 3. The Human Mimic regimen generated yields between three and four times greater than the ISO regimen. The Canadian regimen generated slightly higher yields than the Mimic regimen, whereas the Massachusetts regimen generated lower constituent yields than the mimic regimen. In contrast to regular-yield brand smoking, the Compensatory regimen produced yields much greater than the ISO regimen, and only slightly below the Human Mimic regimen, for Matinee Extra Mild.
Note that the Canadian regimen generated greater constituent yields than either the Massachusetts or the Mimic regimen for the Matinee Extra Mild brand, despite a somewhat lower smoke volume. Although the machine extracted less smoke under the Canadian regimen, this smoke was more concentrated, due to the 100% vent-blocking condition. For example, the concentration of nicotine for the Matinee Extra Mild brand was 66% greater when tested under the Canadian versus the Massachusetts regimen (3.08 versus 2.05 mg/L, respectively).
The nicotine yields produced under the ISO regimen were highly correlated with the Canadian (r = 0.75, P < 0.001), Massachusetts (r = 0.88, P < 0.001), and the Compensatory regimen (r = −86, P < 0.001), but not with the Human Mimic nicotine yields (r = −0.08, P > 0.10). A similar pattern was observed for tar and CO yields.
Measures of Human Nicotine Uptake and Machine Nicotine Yields
Mean salivary cotinine levels were 293.0 ng/mL (SD 135.6) at trial 1, and 321.9 ng/mL (SD 146.8) at trial 2. A linear regression model was run to examine the association between the nicotine yields from each machine smoking regimen and salivary cotinine levels among study participants at trial 1, adjusting for measure of intake and demographic variables. As Table 3 indicates, only the nicotine yields from the Human Mimic regimen were significantly associated with salivary cotinine levels. Similar results were found for trial 2.
Nicotine yields as predictors of saliva cotinine at trial 1 (n = 51)
| Testing regimen . | β* . | t . | P . | Part correlation . | R2 . |
|---|---|---|---|---|---|
| ISO | 0.25 | 1.90 | 0.07 | 0.24 | 0.47 |
| Massachusetts | 0.11 | 0.78 | 0.44 | 0.10 | 0.40 |
| Canadian | 0.17 | 1.21 | 0.23 | 0.16 | 0.45 |
| Compensatory | −0.23 | 1.60 | 0.12 | −0.21 | 0.44 |
| Human Mimic | 0.37 | 2.50 | 0.02 | 0.31 | 0.51 |
| Testing regimen . | β* . | t . | P . | Part correlation . | R2 . |
|---|---|---|---|---|---|
| ISO | 0.25 | 1.90 | 0.07 | 0.24 | 0.47 |
| Massachusetts | 0.11 | 0.78 | 0.44 | 0.10 | 0.40 |
| Canadian | 0.17 | 1.21 | 0.23 | 0.16 | 0.45 |
| Compensatory | −0.23 | 1.60 | 0.12 | −0.21 | 0.44 |
| Human Mimic | 0.37 | 2.50 | 0.02 | 0.31 | 0.51 |
NOTE: Yields from each regimen were entered into separate models. All values are adjusted for gender, time of cotinine sample, and cigarettes per day × total smoke volume.
Standardized β.
Discussion
To our knowledge, this is the first study to evaluate different machine smoking regimens using human smoking behavior and biomarkers of exposure. The results indicate that the yields for the Canadian regimen were near the mean levels of smoke constituents produced under “realistic” machine smoking conditions, which used puffing behavior recorded from actual human smokers. The Massachusetts regimen produced somewhat lower values than the Canadian regimen, whereas the ISO regimen—the current international standard—generated constituent yields well below the Human Mimic yields. Indeed, the Human Mimic yields suggest that study participants were exposed to tar, nicotine, and CO levels that were two to four times greater than the ISO yields.
In their 1936 article, the originators of the Cambridge Filter Method, the predecessor of the ISO and FTC regimens, stated that machine testing regimens should, “sufficiently approximate the conditions of human smoking” (p.836; ref. 7). The results from the current study indicate that the ISO regimen fails this basic criterion. The average smoke volume from the ISO regimen ranked only in the 7th percentile of human smokers for regular-yield brands and fell below the lowest smoke volume drawn by humans from low-yield brands. This is consistent with previous research, which suggests that the puffing variables used by the ISO regimen seriously underestimate the total smoke drawn by human smokers, as well as the flow rate of this volume (3, 13, 24, 33). It should also be noted that the average flow rate for humans observed in the current study was also considerably greater than the ISO puffing variables, as well as the other testing regimens. Flow rate has important implications for filter efficiency, as well as the proportion of diluting air that enters through the porous paper in the tobacco rod and through filter vents (6, 13, 34). As a consequence, flow rates affect the concentration of constituents, as well as the ratio of tar to nicotine. Greater flow rates are also associated with greater depth of inhalation and greater lung exposure to the toxic constituents in tobacco smoke (35, 36).
The Canadian smoking regimen tested cigarettes under the most intensive smoking conditions among the regimens examined in the current study. Nevertheless, the volume of smoke generated by the Canadian regimen was not significantly different than the smoke volume drawn by participants when smoking their regular-yield brands, and below the mean for participants who were switched to the low-yield brand. Thus, although the Canadian regimen is widely considered to produce the maximum emissions to which a smoker is likely to be exposed, the current findings suggest that the Canadian regimen tests brands under puffing conditions that are closer to the mean for human smokers in the current study.
It may seem counterintuitive that the Canadian regimen did not generate greater smoke volume than the Massachusetts regimen when testing the Matinee Extra Mild brand, given the greater intensity of the puffing variables (55 mL puffs and 100% vent blocking versus 44 mL puffs and 50% vent blocking, respectively). However, more intensive puffing and complete ventilation blocking has the effect of increasing the burn rate of the cigarette. In other words, larger puffs that are drawn with the filter ventilation completely blocked consume more of the cigarette rod with each puff and reduce the total burn time of the cigarette. Because the Canadian and Massachusetts regimens draw puffs at the same frequency, the more intensive puffing variables of the Canadian regimen resulted in a lower number of puffs per cigarette as the cigarette rod was consumed more quickly (11.2 versus 13.7 puffs, respectively). Nevertheless, the Canadian regimen generated more concentrated smoke per puff, and produced greater constituent yields than the Massachusetts regimen for every cigarette tested in the current study.
To our knowledge, this is the first time that a compensatory machine smoking regimen has been tested. Of all the regimens examined in the current study, the Compensatory regimen was closest to the human smoke volume for low-yield smoking, although it drastically underestimated smoke volume for regular-yield brands.5
Note that the Compensatory regime proposed by Kozlowski and O'Connor (19) was originally intended for testing lower-yield brands (e.g., <1 mg nicotine), whereas the current study mainly reports results from products with nicotine yields >1 mg.
Perhaps most important, the results underscore the fundamental limitation of machine smoking regimens in predicting measures of human exposure and uptake. The yields from the Massachusetts, Canadian, and the Compensatory regimens were no better at predicting measures of nicotine uptake than were the ISO yields. Indeed, even the Human Mimic nicotine yields, which were derived from actual human puffing behavior, were only moderately correlated with salivary cotinine levels. The relatively modest association between the Human Mimic Yields and salivary cotinine levels reflects the variability in human measures of intake and individual differences in uptake observed among smokers of the same brand. In short, no single nicotine yield can adequately characterize the distribution of nicotine uptake within a single brand. As the current study shows, the limitation of single smoking regimens is present even if the mean puffing variables for each brand can be accurately predicted and replicated by the machine. More generally, the results highlight the fact that it is the smoker—rather than the brand design—that determines nicotine dose and smoke exposure. The needs of the smoker interact with the brand design to determine the puffing behavior required to achieve a particular dose.
For cigarette yields to have any association with human exposure, machine smoking regimens must capture the systematic differences in puffing behavior across product designs that are characteristic of human smoking behavior. Compensatory regimens are the only testing protocols capable of mimicking the interaction between puffing and brand design. Variants on the Compensatory regimen tested in the current study have recently been proposed and warrant further consideration and testing.6
D. Hammond, et al. Revising the ISO machine smoking regime for cigarette emissions: implications for tobacco control policy, submitted for publication.
Limitations
The main limitation of the current study is that the current sample of smokers and cigarette brands is not representative of smokers or all cigarette brands. We selected individuals that smoked brands within a certain tar range and who were not planning to quit in the near future. Future research should replicate the current findings with a broader profile of smokers and cigarette brands. Indeed, analyses examining the association between nicotine uptake and nicotine yields only include brands with ISO tar yields between 10 and 14 mg. Although this range accounts for a considerable proportion of the Canadian market share, future analyses should include brands that represent the available spectrum of designs and emission profiles.
A second limitation concerns the discrepancy between the mean number of puffs recorded from participants and the number taken by the Filtrona machine when conducting the Human Mimic regimen. This discrepancy generated ∼11% more smoke for regular-yield brands and 9% less smoke for the low-yield brand compared with mean smoke volumes recorded from participants. As a result, the Mimic values for regular-yield brands may be slightly exaggerated, whereas the low-yield values may represent a slight underestimate. Ideally, Human Mimic regimens should be conducted on puff duplicators that model the puffing profile of smokers more precisely, similar to those used by the tobacco industry (37).
A third limitation concerns vent blocking. Measures of vent blocking were not collected from participants in the field study. As a result, the vent-blocking conditions for the Human Mimic testing were set at 50%, based on the few population-based studies that have been conducted (32). This limitation is mitigated somewhat by the fact that most of the regular-yield brands included in the study had lower levels of ventilation. For example, the three most popular brands smoked by 17 of the participants had a mean ventilation level of only 5%. As a result, lip and finger placement on the filter would have had little or no effect on the levels of tar, nicotine, or carbon monoxide delivered to participants. However, measures of vent blocking would have been informative in assessing compensation to the ventilated Matinee Extra Mild brand in the low-yield condition. It should also be noted that using the CReSSmicro device may interfere with naturalistic vent blocking. When cigarettes are inserted into the device, the perforations on the cigarette filter sit immediately outside the mouthpiece. Participants may find it awkward to grip the filter directly, and those who do may not obscure the vents in the usual fashion. One consequence is that low-yield smokers may have increased the intensity of their puffing behavior somewhat to compensate for the diluted smoke from the unblocked vents.
Conclusions
Overall, the current findings indicate that none of the smoking regimens currently in use adequately “represent” human smoking behavior and none are significantly associated with measures of nicotine uptake among human participants. The results highlight the fact that no single machine testing regimen is capable of predicting individual exposure. It should be noted that the Canadian regimen was never intended to represent some average of human smoking, but to establish intensive smoking conditions. Nevertheless, these findings have important implications for using the yields as consumer information. In many countries, regulators require manufacturers to print cigarette yields on packages; given that cigarette yields are not associated with human exposure and cannot be used by individual smokers to compare brands in any meaningful way, there is little or no reason to communicate numerical yields to consumers. Indeed, standard smoking regimens introduce deceptive differences between brands that, when communicated directly to smokers, are often misunderstood and misused (38). Adding a higher range of numbers from more intensive smoking regimens—as is currently the practice in Canada—does not provide any additional information that is meaningful to individual smokers. As a consequence, regulators should remove numerical cigarette yields from packages, as recommended by the WHO Study Group on Tobacco Regulation (20).
The limitations of machine smoking regimens have led many to question the utility of such testing protocols. Despite their limitations, constituent yields help us to understand the chemical profile in tobacco smoke and how this profile varies under different smoking conditions. Although cigarette yields are not measures of exposure and tell us nothing about human uptake of smoke constituents, cigarette yields may serve as a useful mechanism for mandating changes to cigarette design. However, the effectiveness of emission-based regulations depends on the magnitude of the reductions in smoke constituents. Using yields to make precise distinctions between brands currently on the market is unlikely to have any public health benefit. Indeed, the standard smoking regimens (i.e., the ISO, Massachusetts, and Canadian regimens) are ill-suited for this purpose. For example, the current European Union legislation that sets maximum limits of 10 mg tar, 1 mg nicotine, and 10 mg carbon monoxide under the ISO regimen, seems to have had little effect, other than to increase the levels of filter ventilation among European Union brands (39). Only limits that reduce cigarette yields well below current market standards or reduce the reinforcing properties of cigarettes are likely to have a significant effect on health.
The ISO/FTC smoking regimen has been retained by regulators throughout the world in the absence of any suitable replacement. As internal BAT documents note, “Governments and their associated laboratories are aware of compensation but are reluctant to change since they can offer no viable alternative to the present smoking regimen”(40). As regulators contemplate adopting alternative machine smoking regimens, we would urge them to also consider a more comprehensive set of testing regulations, including biological measures of exposure and mandatory reporting guidelines for cigarette design variables, such as filter ventilation, pressure drop, filter efficiency, tobacco blend, and additives. The financial responsibility for conducting testing should be borne by tobacco manufacturers. However, the actual testing should be done by reliable independent testing authorities who have no financial links to the tobacco industry.
Grant support: U.S. National Cancer Institute/NIH (Roswell Park Transdisciplinary Tobacco Use Research Center, P50 CA111236, and R01 CA100362), the American Cancer Society, Health Canada, the Canadian Institutes for Health Research, and the Department of Health (England).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Note: This study was conducted at the University of Waterloo.
Acknowledgments
We thank Mary-Jean Costello and Tara Elton-Marshall at the University of Waterloo for their help in conducting this research, and Warren Davis at Roswell Park Cancer Institute for arranging the salivary cotinine testing.